Chemistry Cheat Sheet
ADVERTISEMENT
200 Ways to Pass the Chemistry
Physical Setting Regents Exam
1. Protons are positively charged (+).
2. Neutrons have no charge.
3. Electrons are small and are negatively charged (-).
4. Protons & neutrons are in an atom’s nucleus (nucleons).
5. Electrons are found in “clouds” (orbitals) around an atom’s nucleus.
6. The mass number is equal to an atom’s number of protons and neutrons added
together.
7. The atomic number is equal to the number of protons in the nucleus of an atom.
8. The number of neutrons = mass number – atomic number.
9. Isotopes are atoms with equal numbers of protons, but differ in their neutron
numbers.
10. Cations are positive (+) ions and form when a neutral atom loses electrons. They
are smaller than their parent atom.
11. Anions are negative ions and form when a neutral atom gains electrons. They are
larger than their parent atom.
12. Ernest Rutherford’s gold foil experiment showed that an atom is mostly empty
space with a small, dense, positively-charged nucleus.
13. J.J. Thompson discovered the electron and developed the “plum-pudding” model
of the atom.
+ - + -
Positive & negative
+ - + - +
particles spread throughout
- + - +
entire atom.
-
14. Dalton’s model of the atom was a solid sphere of matter that was uniform
throughout.
15. The Bohr Model of the atom placed electrons in “planet-like” orbits around the
nucleus of an atom.
16. The current, wave-mechanical model of the atom has electrons in “clouds”
(orbitals) around the nucleus.
17. USE THE REFERENCE TABLES!!!
18. “STP” means “Standard Temperature and Pressure.” (273 Kelvin & 1 atm)
19. Electrons emit energy as light when they jump from higher energy levels back
down to lower (ground state) energy levels. Bright line spectra are produced.
20. Elements are pure substances composed of only one kind of atom.
21. Binary compounds are substances made up of only two kinds of atoms.
(examples: H
O, NH
, CO
)
2
3
2
22. Diatomic molecules are elements that form two atom molecules in their natural
“BrINClHOF”
form at STP. Remember the phrase –
(Br
, I
, N
, CL
, H
, O
, F
)
2
2
2
2
2
2
2
23. Use this diagram to help determine the number of significant figures in a
measured value…
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2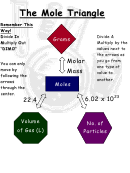 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12








