Observing Phase Changes Science Worksheets
ADVERTISEMENT
P a g e
| 1
Name_________________________________________
Observing Phase Changes
Purpose: To observe changes in energy as water changes state Background Information: Heat changes the temperature
of a substance. When heat is absorbed by a substance, the kinetic energy is of the substance is increased; the particles
(atoms and molecules) of the substance begin to speed up and move faster. The reverse is also true. When a substance
cools off, it releases, or loses, heat and the kinetic energy decreases. The particles slow down. Heat changes the state of
matter. A substance will change from one state or phase to another at specific combinations of temperature and
surrounding pressure. Usually, the pressure is atmospheric pressure, so temperature is the determining factor to the
change in state in those cases. There are 3 states of water which are liquid, solid and gas. All three states exist on earth.
There are names for each of the phase changes of water. They are:
Water going from a solid to a liquid: Melting – absorbs heat
Water going from a liquid to a gas: Evaporation – absorbs heat
Water going from a solid to a gas: Sublimation – absorbs heat
Water going from a liquid to a solid: Freezing – releases heat
Water going from a gas to a liquid: Condensation – releases heat
Water going from a gas to a solid: Deposition – releases heat
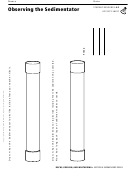
Prediction: When heat is added to ice, the ice will change state from solid, to liquid, to gas. What do you think a graph of
this data will show? Sketch it below:
Time
M. Poarch 2015
science-class.net
Permission to copy granted for non-profit, educational use only.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4