Phase Change Diagram (Energy Removed From Substance)
ADVERTISEMENT
Name ____________________________ Date _________ Period ___
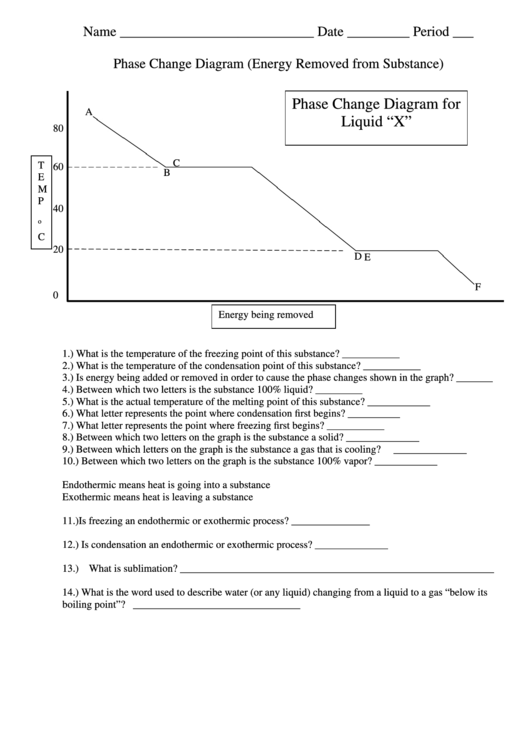
Phase Change Diagram (Energy Removed from Substance)
Phase Change Diagram for
A
Liquid “X”
80
C
T
60
B
E
M
P
40
o
C
20
D
E
F
0
Energy being removed
1.) What is the temperature of the freezing point of this substance? ___________
2.) What is the temperature of the condensation point of this substance? ___________
3.) Is energy being added or removed in order to cause the phase changes shown in the graph? _______
4.) Between which two letters is the substance 100% liquid? _________
5.) What is the actual temperature of the melting point of this substance? ____________
6.) What letter represents the point where condensation first begins? __________
7.) What letter represents the point where freezing first begins? ___________
8.) Between which two letters on the graph is the substance a solid? ______________
9.) Between which letters on the graph is the substance a gas that is cooling?
______________
10.) Between which two letters on the graph is the substance 100% vapor? ____________
Endothermic means heat is going into a substance
Exothermic means heat is leaving a substance
11.)Is freezing an endothermic or exothermic process? _______________
12.) Is condensation an endothermic or exothermic process? ______________
13.) What is sublimation? ____________________________________________________________
14.) What is the word used to describe water (or any liquid) changing from a liquid to a gas “below its
boiling point”? ________________________________
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1