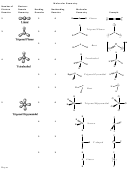
Molecular Polarity Chart
ADVERTISEMENT
A. Romero 2009
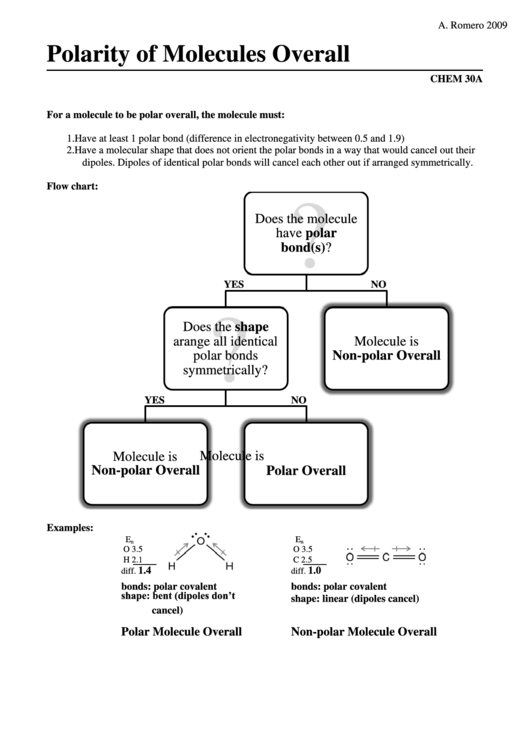
Polarity of Molecules Overall
CHEM 30A
For a molecule to be polar overall, the molecule must:
1. Have at least 1 polar bond (difference in electronegativity between 0.5 and 1.9)
2. Have a molecular shape that does not orient the polar bonds in a way that would cancel out their
dipoles. Dipoles of identical polar bonds will cancel each other out if arranged symmetrically.
Flow chart:
?
Does the molecule
have polar
bond(s)?
YES
NO
?
Does the shape
arange all identical
Molecule is
polar bonds
Non-polar Overall
symmetrically?
YES
NO
Molecule is
Molecule is
Non-polar Overall
Polar Overall
Examples:
E
E
n
n
O 3.5
O 3.5
H 2.1
C 2.5
1.4
1.0
diff.
diff.
bonds: polar covalent
bonds: polar covalent
shape: bent (dipoles don’t
shape: linear (dipoles cancel)
cancel)
Polar Molecule Overall
Non-polar Molecule Overall
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1