Ap Chemistry: Inorganic Chemical Nomenclature Reference Chart
ADVERTISEMENT
AP Chemistry: Inorganic Chemical Nomenclature Reference Chart
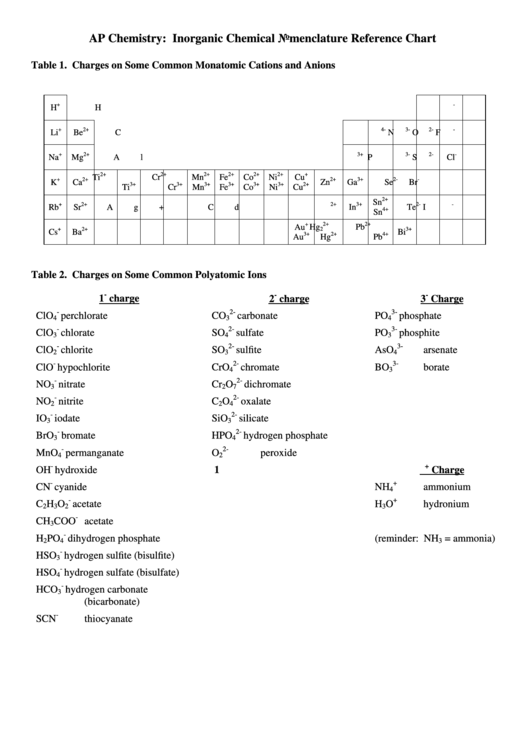
Table 1. Charges on Some Common Monatomic Cations and Anions
+
-
H
H
+
2+
4-
3-
2-
-
Li
Be
C
N
O
F
+
2+
3+
3-
2-
-
Na
Mg
Al
P
S
Cl
2+
2+
2+
2+
2+
2+
+
Ti
Cr
Mn
Fe
Co
Ni
Cu
+
2+
2+
3+
2-
-
K
Ca
Zn
Ga
Se
Br
3+
3+
3+
3+
3+
3+
2+
Ti
Cr
Mn
Fe
Co
Ni
Cu
2+
Sn
+
2+
2+
3+
2-
-
Rb
Sr
Ag+
Cd
In
Te
I
4+
Sn
+
2+
2+
Au
Hg
Pb
+
2+
2
3+
Cs
Ba
Bi
3+
2+
4+
Au
Hg
Pb
Table 2. Charges on Some Common Polyatomic Ions
-
-
-
1
charge
2
charge
3
Charge
-
2-
3-
ClO
perchlorate
CO
carbonate
PO
phosphate
4
3
4
-
2-
3-
ClO
chlorate
SO
sulfate
PO
phosphite
3
4
3
-
2-
3-
ClO
chlorite
SO
sulfite
AsO
arsenate
2
3
4
-
2-
3-
ClO
hypochlorite
CrO
chromate
BO
borate
4
3
-
2-
NO
nitrate
Cr
O
dichromate
3
2
7
-
2-
NO
nitrite
C
O
oxalate
2
2
4
-
2-
IO
iodate
SiO
silicate
3
3
-
2-
BrO
bromate
HPO
hydrogen phosphate
3
4
-
2-
MnO
permanganate
O
peroxide
4
2
-
+
OH
hydroxide
1
Charge
-
+
CN
cyanide
NH
ammonium
4
-
+
C
H
O
acetate
H
O
hydronium
2
3
2
3
-
CH
COO
acetate
3
-
H
PO
dihydrogen phosphate
(reminder: NH
= ammonia)
2
4
3
-
HSO
hydrogen sulfite (bisulfite)
3
-
HSO
hydrogen sulfate (bisulfate)
4
-
HCO
hydrogen carbonate
3
(bicarbonate)
-
SCN
thiocyanate
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3








