Table Of Equilibrium Constants
ADVERTISEMENT
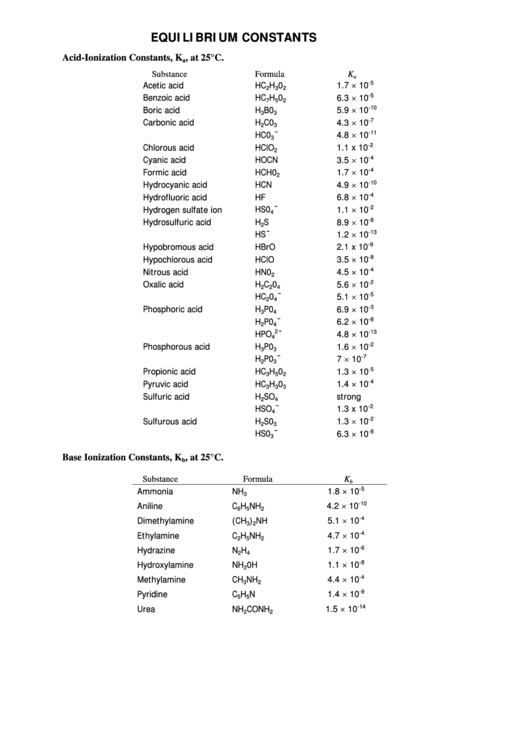
EQUILIBRIUM CONSTANTS
Acid-Ionization Constants, K
, at 25°C.
a
Substance
Formula
K
a
-5
Acetic acid
HC
H
0
1.7
10
2
3
2
-5
Benzoic acid
HC
H
0
6.3
10
7
5
2
-10
Boric acid
H
B0
5.9
10
3
3
-7
Carbonic acid
H
C0
4.3
10
2
3
HC0
-11
4.8
10
3
-2
Chlorous acid
HClO
1.1 x 10
2
-4
Cyanic acid
HOCN
3.5
10
-4
Formic acid
HCH0
1.7
10
2
-10
Hydrocyanic acid
HCN
4.9
10
-4
Hydrofluoric acid
HF
6.8
10
Hydrogen sulfate ion
HS0
-2
1.1
10
4
Hydrosulfuric acid
H
S
8.9
10
-8
2
-13
HS
1.2
10
-9
Hypobromous acid
HBrO
2.1 x 10
-8
Hypochlorous acid
HClO
3.5
10
-4
Nitrous acid
HN0
4.5
10
2
Oxalic acid
H
C
0
-2
5.6
10
2
2
4
HC
0
5.1
10
-5
2
4
Phosphoric acid
H
P0
6.9
10
-3
3
4
H
P0
-8
6.2
10
2
4
HPO
2
-13
4.8
10
4
-2
Phosphorous acid
H
P0
1.6
10
3
3
H
P0
-7
7
10
2
3
Propionic acid
HC
H
0
1.3
10
-5
3
5
2
Pyruvic acid
HC
H
0
1.4
10
-4
3
3
3
Sulfuric acid
H
SO
strong
2
4
HSO
-2
1.3 x 10
4
-2
Sulfurous acid
H
S0
1.3
10
2
3
HS0
-8
6.3
10
3
Base Ionization Constants, K
, at 25°C.
b
Substance
Formula
K
b
Ammonia
NH
1.8
10
-5
3
Aniline
C
H
NH
4.2
10
-10
6
5
2
-4
Dimethylamine
(CH
)
NH
5.1
10
3
2
-4
Ethylamine
C
H
NH
4.7
10
2
5
2
Hydrazine
N
H
1.7
10
-6
2
4
-8
Hydroxylamine
NH
0H
1.1
10
2
-4
Methylamine
CH
NH
4.4
10
3
2
Pyridine
C
H
N
1.4
10
-9
5
5
-14
Urea
NH
CONH
1.5
10
2
2
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1