Selected Thermochemical Values - Vaxasoftware
ADVERTISEMENT
Selected thermochemical values
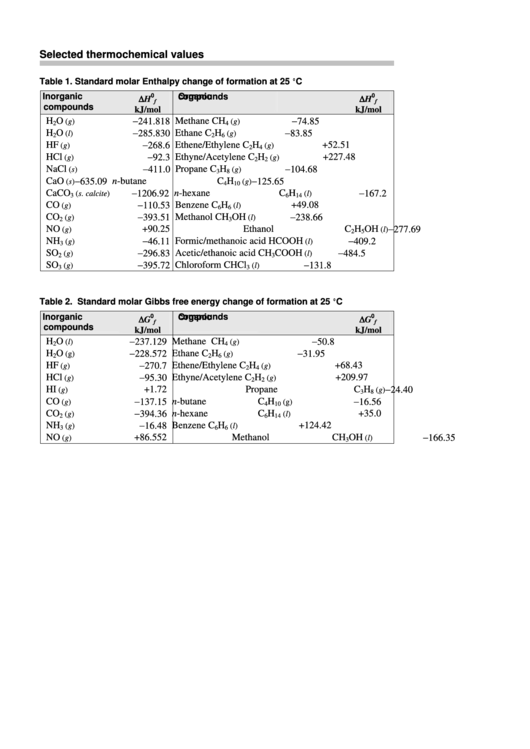
Table 1. Standard molar Enthalpy change of formation at 25 °C
Inorganic
∆H
Organic
∆H
0
0
f
f
compounds
compounds
kJ/mol
kJ/mol
−241.818 Methane
−74.85
H
O
CH
(g)
(g)
2
4
−285.830 Ethane
−83.85
H
O
C
H
(l)
(g)
2
2
6
−268.6 Ethene/Ethylene
HF
C
H
+52.51
(g)
(g)
2
4
−92.3 Ethyne/Acetylene
HCl
C
H
+227.48
(g)
(g)
2
2
−411.0 Propane
−104.68
NaCl
C
H
(s)
(g)
3
8
−635.09 n-butane
−125.65
CaO
C
H
(s)
(g)
4
10
−1 206.92 n-hexane
−167.2
CaCO
C
H
(s. calcite)
(l)
3
6
14
−110.53 Benzene
CO
C
H
+49.08
(g)
(l)
6
6
−393.51 Methanol
−238.66
CO
CH
OH
(g)
(l)
2
3
−277.69
NO
+90.25 Ethanol
C
H
OH
(g)
(l)
2
5
−46.11 Formic/methanoic acid
−409.2
NH
HCOOH
(g)
(l)
3
−296.83 Acetic/ethanoic acid
−484.5
SO
CH
COOH
(g)
(l)
2
3
−395.72 Chloroform
−131.8
SO
CHCl
(g)
(l)
3
3
Table 2. Standard molar Gibbs free energy change of formation at 25 °C
Inorganic
∆G
Organic
∆G
0
0
f
f
compounds
compounds
kJ/mol
kJ/mol
−237.129
−50.8
H
O
Methane
CH
(l)
(g)
2
4
−228.572
−31.95
H
O
Ethane
C
H
(g)
(g)
2
2
6
−270.7
HF
Ethene/Ethylene
C
H
+68.43
(g)
(g)
2
4
−95.30
HCl
Ethyne/Acetylene
C
H
+209.97
(g)
(g)
2
2
−24.40
HI
+1.72
Propane
C
H
(g)
(g)
3
8
−137.15
−16.56
CO
n-butane
C
H
(g)
(g)
4
10
−394.36
CO
n-hexane
C
H
+35.0
(g)
(l)
2
6
14
−16.48
NH
Benzene
C
H
+124.42
(g)
(l)
3
6
6
−166.35
NO
+86.552
Methanol
CH
OH
(g)
(l)
3
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2








