Selective Precipitation - Winston Knoll Collegiate
ADVERTISEMENT
Selective Precipitation.
If several ions (cations or anions) are together in solution, they may be
systematically removed using a process known as selective precipitation.
If cations are in solution, anions must be added in an order that will remove the
cations one at a time by forming an insoluble precipitate.
Consider a solution containing the anions carbonate and chloride.
First consider all of the cations that are insoluble with each of these anions:
CO 3 2- (Ba 2+ , Ca 2+ , Cu 2+ , Fe 2+ ,Pb , Mg 2+ , Hg , Ag + , Zn 2+ )
Cl - (Hg 2 2+ , Ag + )
If, for example, the zinc cation is added, the carbonate will precipitate out and
leave the chloride ion in solution. With one ion removed, either cation that is
insoluble with chloride may be added to remove the second anion.
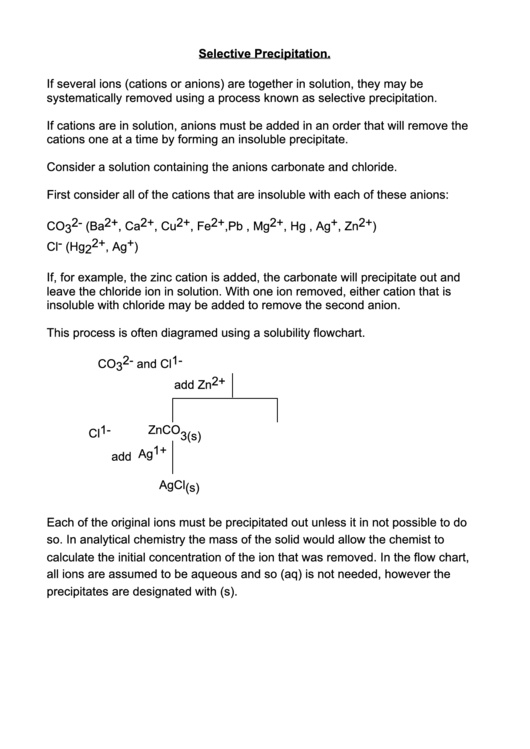
This process is often diagramed using a solubility flowchart.
CO 3 2- and Cl 1-
add Zn 2+
Cl 1-
ZnCO
3(s)
1+
add Ag
AgCl (s)
Each of the original ions must be precipitated out unless it in not possible to do
so. In analytical chemistry the mass of the solid would allow the chemist to
calculate the initial concentration of the ion that was removed. In the flow chart,
all ions are assumed to be aqueous and so (aq) is not needed, however the
precipitates are designated with (s).
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2








