The Mole Highway
ADVERTISEMENT
H 201 K. Bailey
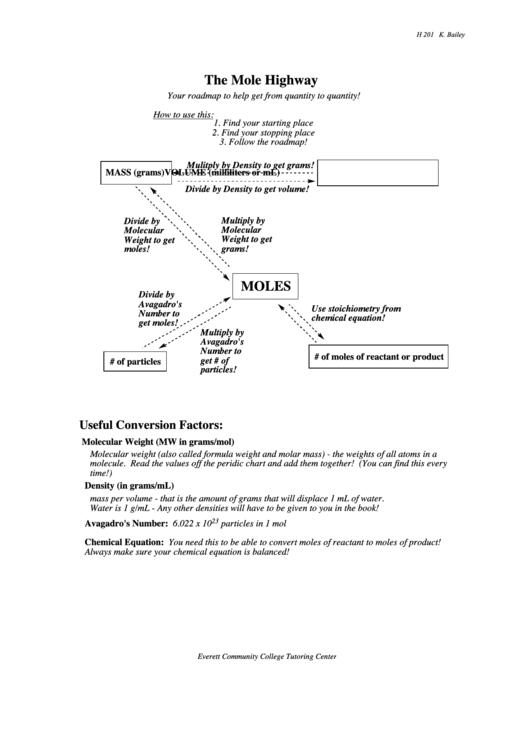
The Mole Highway
Your roadmap to help get from quantity to quantity!
How to use this:
1. Find your starting place
2. Find your stopping place
3. Follow the roadmap!
Mulitply by Density to get grams!
MASS (grams)
VOLUME (milliliters or mL)
Divide by Density to get volume!
Multiply by
Divide by
Molecular
Molecular
Weight to get
Weight to get
grams!
moles!
MOLES
Divide by
Avagadro's
Use stoichiometry from
Number to
chemical equation!
get moles!
Multiply by
Avagadro's
Number to
# of moles of reactant or product
get # of
# of particles
particles!
Useful Conversion Factors:
Molecular Weight (MW in grams/mol)
Molecular weight (also called formula weight and molar mass) - the weights of all atoms in a
molecule. Read the values off the peridic chart and add them together! (You can find this every
time!)
Density (in grams/mL)
mass per volume - that is the amount of grams that will displace 1 mL of water.
Water is 1 g/mL - Any other densities will have to be given to you in the book!
23
Avagadro's Number: 6.022 x 10
particles in 1 mol
Chemical Equation: You need this to be able to convert moles of reactant to moles of product!
Always make sure your chemical equation is balanced!
Everett Community College Tutoring Center
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1