Periodic Table Worksheet
ADVERTISEMENT
Name: __________________________________________________
Homework 2
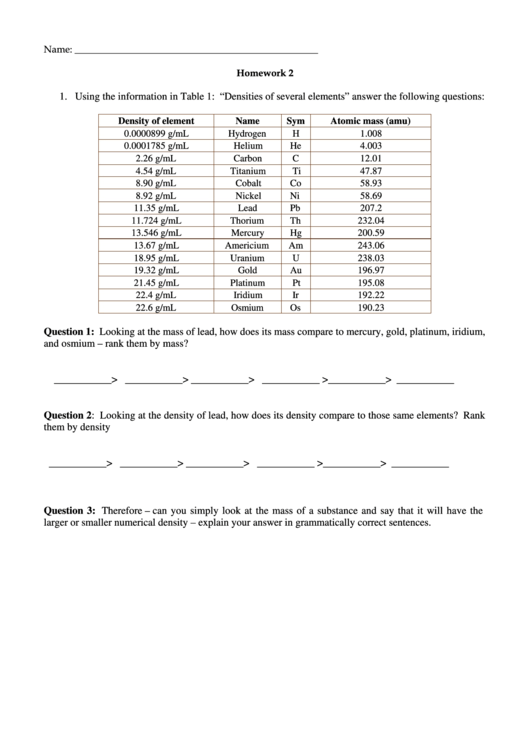
1. Using the information in Table 1: “Densities of several elements” answer the following questions:
Density of element
Name
Sym
Atomic mass (amu)
0.0000899 g/mL
Hydrogen
H
1.008
0.0001785 g/mL
Helium
He
4.003
2.26 g/mL
Carbon
C
12.01
4.54 g/mL
Titanium
Ti
47.87
8.90 g/mL
Cobalt
Co
58.93
8.92 g/mL
Nickel
Ni
58.69
11.35 g/mL
Lead
Pb
207.2
11.724 g/mL
Thorium
Th
232.04
13.546 g/mL
Mercury
Hg
200.59
13.67 g/mL
Americium
Am
243.06
18.95 g/mL
Uranium
U
238.03
19.32 g/mL
Gold
Au
196.97
21.45 g/mL
Platinum
Pt
195.08
22.4 g/mL
Iridium
Ir
192.22
22.6 g/mL
Osmium
Os
190.23
Question 1: Looking at the mass of lead, how does its mass compare to mercury, gold, platinum, iridium,
and osmium – rank them by mass?
___________> ___________> ___________> ___________ >___________> ___________
Question 2: Looking at the density of lead, how does its density compare to those same elements? Rank
them by density
___________> ___________> ___________> ___________ >___________> ___________
Question 3: Therefore – can you simply look at the mass of a substance and say that it will have the
larger or smaller numerical density – explain your answer in grammatically correct sentences.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4








