Radioactive Decay Worksheet
ADVERTISEMENT
Radioactive Decay Series
Integrated Science 3 Honors
Name ______________________________ Per. _____
Background
Radioactive material contains atoms whose nuclei are unstable. These atoms, consequently, are likely to 'fall
apart.' When radioactive atoms fall apart, or decay, they release energy and/or particles in the form of ionizing
radiation. When particles are released, the composition of the atomic nucleus changes. In the process this produces a
different atom and, consequently, a different element. In many cases, this new element is also unstable and will decay
by emitting radioactive particles. This process, called a radioactive decay chain, will continue until a stable atomic
nucleus (a stable isotope of the new element) is reached.
The rate of decay at each step of the process is a characteristic property of the specific element/isotope. This is
expressed as the half-life, the amount of time required for one-half of the atoms in a given sample to decay. Half-lives
of specific elements/isotopes vary from seconds to millions of years.
Radioactive elements, and radioactive isotopes of stable elements, are found in nature. As these naturally
occurring elements decay, they progress through a decay series until reaching a stable end-product. This naturally
occurring radioactive decay is responsible for much of the 'background' radiation which each of us receives. Humans
have developed the capability to produce 'man-made' radioactive elements as well. These products, associated with
nuclear weapons production, nuclear power production and nuclear medicine, also follow characteristic decay series
having characteristic decay rates. In this activity we will look at some natural radioactive decay series.
Procedure
1.
Use the Table of Atomic Transitions and the Chart of the Isotopes as an example to follow and understand the
radioactive decay series of thorium-232.
2.
Use the Table of Atomic Transitions to plot the radioactive decay series for Uranium-235 on the Chart of the
Isotopes.
3.
Use the Chart of the Isotopes to complete the Table of Atomic Transitions for Uranium-238.
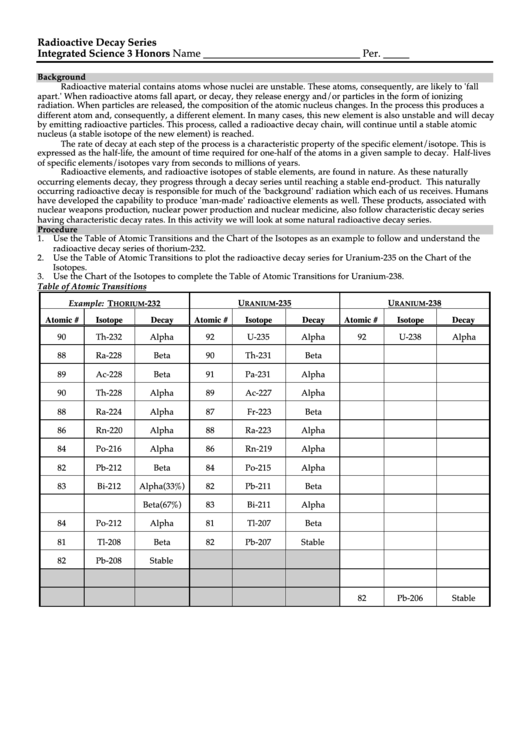
Table of Atomic Transitions
Example: T
-232
U
-235
U
-238
HORIUM
RANIUM
RANIUM
Atomic #
Isotope
Decay
Atomic #
Isotope
Decay
Atomic #
Isotope
Decay
90
Th-232
Alpha
92
U-235
Alpha
92
U-238
Alpha
88
Ra-228
Beta
90
Th-231
Beta
89
Ac-228
Beta
91
Pa-231
Alpha
90
Th-228
Alpha
89
Ac-227
Alpha
88
Ra-224
Alpha
87
Fr-223
Beta
86
Rn-220
Alpha
88
Ra-223
Alpha
84
Po-216
Alpha
86
Rn-219
Alpha
82
Pb-212
Beta
84
Po-215
Alpha
83
Bi-212
Alpha(33%)
82
Pb-211
Beta
Beta(67%)
83
Bi-211
Alpha
84
Po-212
Alpha
81
Tl-207
Beta
81
Tl-208
Beta
82
Pb-207
Stable
82
Pb-208
Stable
82
Pb-206
Stable
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1








