Subatomic Particles For Atoms, Ions And Isotopes - Mr. Birrell
ADVERTISEMENT
Name:_________________________________________________________
Date:____________________________
Subatomic Particles for Atoms, Ions and Isotopes-Practice
Use your periodic table to complete this worksheet.
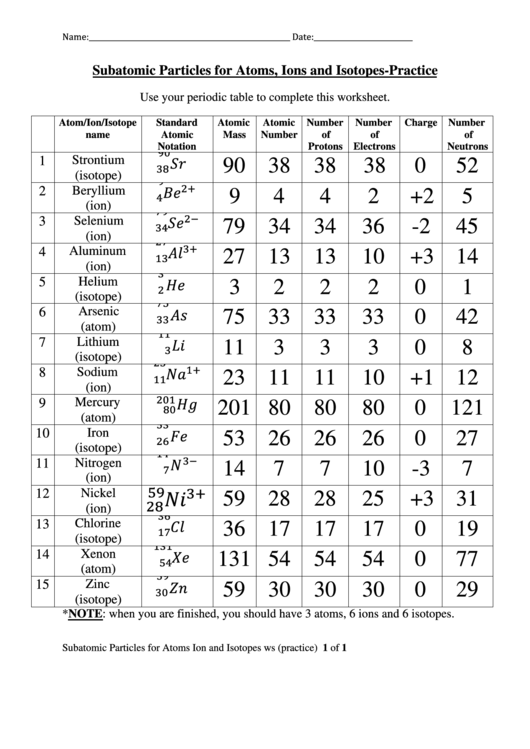
Atom/Ion/Isotope
Standard
Atomic
Atomic
Number
Number
Charge
Number
name
Atomic
Mass
Number
of
of
of
Notation
Protons
Electrons
Neutrons
Strontium
1
90 38
38
38
0
52
(isotope)
2
Beryllium
9
4
4
2
+2
5
(ion)
Selenium
3
79 34
34
36
-2
45
(ion)
4
Aluminum
27 13
13
10
+3 14
(ion)
Helium
5
3
2
2
2
0
1
(isotope)
Arsenic
6
75 33
33
33
0
42
(atom)
7
Lithium
11
3
3
3
0
8
(isotope)
Sodium
8
23 11
11
10
+1 12
(ion)
Mercury
9
201 80
80
80
0
121
(atom)
10
Iron
53 26
26
26
0
27
(isotope)
Nitrogen
11
14
7
7
10
-3
7
(ion)
Nickel
12
59 28
28
25
+3 31
(ion)
13
Chlorine
36 17
17
17
0
19
(isotope)
Xenon
14
131 54
54
54
0
77
(atom)
15
Zinc
59 30
30
30
0
29
(isotope)
*NOTE: when you are finished, you should have 3 atoms, 6 ions and 6 isotopes.
Subatomic Particles for Atoms Ion and Isotopes ws (practice) KEY.docx
Page 1 of 1
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1








