Chemistry Math Reference Sheet
ADVERTISEMENT
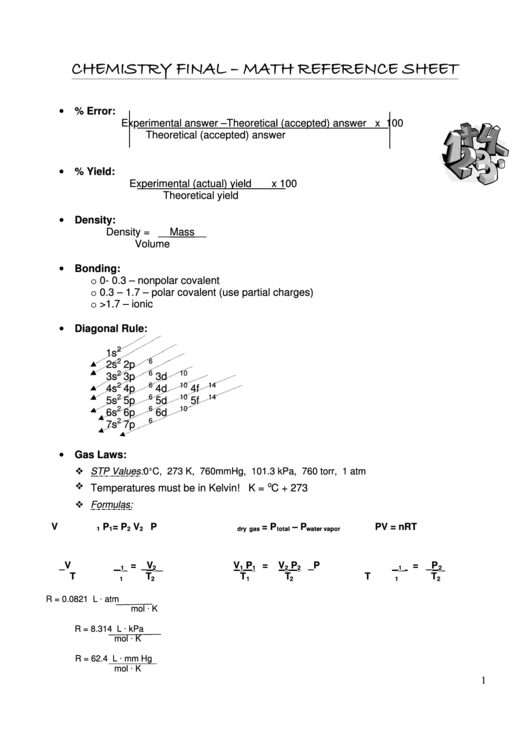
CHEMISTRY FINAL – MATH REFERENCE SHEET
• % Error:
Experimental answer –Theoretical (accepted) answer x 100
Theoretical (accepted) answer
• % Yield:
Experimental (actual) yield
x 100
Theoretical yield
• Density:
Density = __Mass__
Volume
• Bonding:
o 0- 0.3 – nonpolar covalent
o 0.3 – 1.7 – polar covalent (use partial charges)
o >1.7 – ionic
• Diagonal Rule:
2
1s
2
6
2s
2p
2
6
10
3s
3p
3d
2
6
10
14
4s
4p
4d
4f
2
6
10
14
5s
5p
5d
5f
2
6
10
6s
6p
6d
2
6
7s
7p
• Gas Laws:
STP Values: 0°C, 273 K, 760mmHg, 101.3 kPa, 760 torr, 1 atm
o
Temperatures must be in Kelvin! K =
C + 273
Formulas:
V
P
= P
V
P
= P
– P
PV = nRT
1
1
2
2
dry gas
total
water vapor
_V
= _V
V
P
=
V
P
_P
= _P
1_
2__
1
1
2
2
1_
2_
T
T
T
T
T
T
1
2
1
2
1
2
R = 0.0821 L · atm
mol · K
R = 8.314 L · kPa
mol · K
R = 62.4 L · mm Hg
mol · K
1
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3