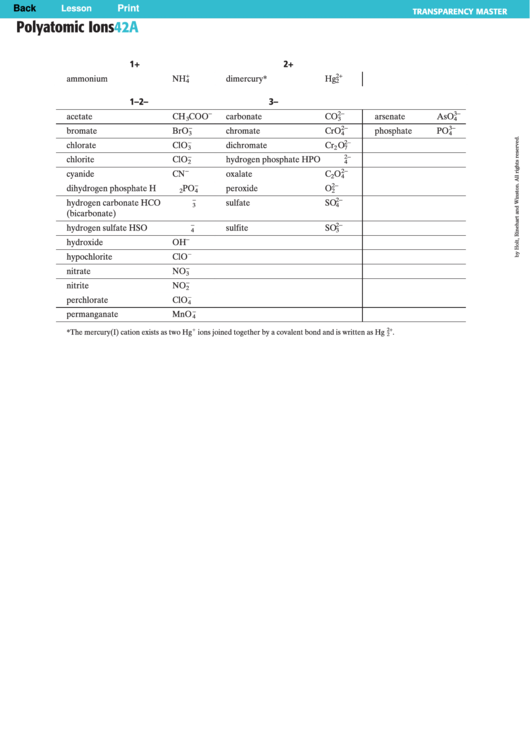
Polyatomic Ions Chart
ADVERTISEMENT
Back
Lesson
Print
TRANSPARENCY MASTER
Polyatomic Ions
42A
1+
2+
+
2+
ammonium
NH
dimercury*
Hg
4
2
1–
2–
3–
−
2−
3−
acetate
CH
COO
carbonate
CO
arsenate
AsO
3
3
4
−
2−
3−
bromate
BrO
chromate
CrO
phosphate
PO
3
4
4
−
2−
chlorate
ClO
dichromate
Cr
O
3
2
7
−
2−
chlorite
ClO
hydrogen phosphate
HPO
2
4
−
2−
cyanide
CN
oxalate
C
O
2
4
−
2−
dihydrogen phosphate
H
PO
peroxide
O
2
4
2
−
2−
hydrogen carbonate
HCO
sulfate
SO
3
4
(bicarbonate)
−
2−
hydrogen sulfate
HSO
sulfite
SO
4
3
−
hydroxide
OH
−
hypochlorite
ClO
−
nitrate
NO
3
−
nitrite
NO
2
−
perchlorate
ClO
4
−
permanganate
MnO
4
+
2+
*The mercury(I) cation exists as two Hg
ions joined together by a covalent bond and is written as Hg
.
2
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1








