How To Create An Electronic Regulatory Binder In Sharepoint Page 4
ADVERTISEMENT
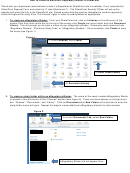
Attachment 2: Examples of sub-folders for you eRegulatory Binder. Please create sub-folders
applicable to your study.
•
Current Protocol
•
Outdated Protocols
•
Current ICF & HIPAA Authorization Form
•
Outdated ICF & HIPAA Authorization Form
•
Investigator’s Brochure
•
Investigator’s Agreement
•
IRB Correspondence (IRB submissions and letters)
•
Other Committee Correspondence
•
Delegation of Authority Log
•
FDA Form 1572
•
Financial Disclosure Forms
•
CVs, Licenses, Training Certificates for PI, Sub-Is, Key Personnel
•
Protocol Deviation Log
•
Serious Adverse Events (SAEs)
•
IND/IDE Safety Reports
•
Recruitment
•
Laboratory Certification
•
Subject Enrollment/Screening Log
•
Site Visit Log
•
Specimen Sample Logs
•
Pharmaceutical Information
•
Case Report Forms
•
Sponsor Correspondence
•
General Correspondence
For a more detailed list of sub-folders email: RSCMonitoringUnit@uams.edu.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4








