Revision Guide Alkenes 3.4 - Chemistry Sheet
ADVERTISEMENT
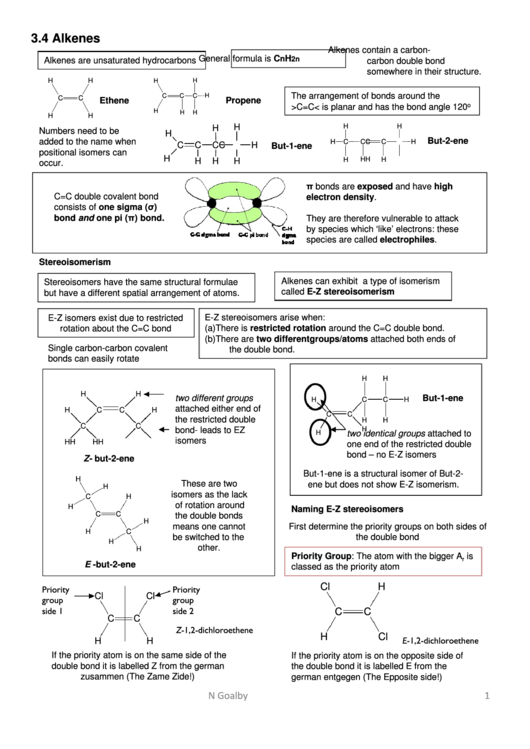
3.4 Alkenes
Alkenes contain a carbon-
General formula is C
H
n
2n
Alkenes are unsaturated hydrocarbons
carbon double bond
somewhere in their structure.
H
H
H
H
The arrangement of bonds around the
C
C
C
H
C
C
Ethene
Propene
o
>C=C< is planar and has the bond angle 120
H
H
H
H
H
H
H
H
H
Numbers need to be
H
But-2-ene
added to the name when
H
C
C
C
C
H
C
C
C
C
H
But-1-ene
positional isomers can
H
H
H
H
H
H
H
H
occur.
π bonds are exposed and have high
C=C double covalent bond
electron density.
consists of one sigma (σ)
bond and one pi (π) bond.
They are therefore vulnerable to attack
by species which ‘like’ electrons: these
species are called electrophiles.
Stereoisomerism
Alkenes can exhibit a type of isomerism
Stereoisomers have the same structural formulae
called E-Z stereoisomerism
but have a different spatial arrangement of atoms.
E-Z stereoisomers arise when:
E-Z isomers exist due to restricted
rotation about the C=C bond
(a)
There is restricted rotation around the C=C double bond.
(b)
There are two different groups/atoms attached both ends of
Single carbon-carbon covalent
the double bond.
bonds can easily rotate
H
H
H
H
two different groups
But-1-ene
H
C
C
H
attached either end of
H
C
C
H
C
C
the restricted double
H
H
C
C
bond- leads to EZ
H
H
two identical groups attached to
isomers
H
H
H
H
one end of the restricted double
bond – no E-Z isomers
Z- but-2-ene
But-1-ene is a structural isomer of But-2-
H
These are two
ene but does not show E-Z isomerism.
H
isomers as the lack
C
H
of rotation around
H
Naming E-Z stereoisomers
C
C
the double bonds
H
means one cannot
First determine the priority groups on both sides of
H
C
the double bond
be switched to the
H
other.
H
Priority Group: The atom with the bigger A
is
r
E -but-2-ene
classed as the priority atom
Cl
H
Priority
Priority
Cl
Cl
group
group
side 1
side 2
C
C
C
C
Z-1,2-dichloroethene
H
Cl
E-1,2-dichloroethene
H
H
If the priority atom is on the same side of the
If the priority atom is on the opposite side of
double bond it is labelled Z from the german
the double bond it is labelled E from the
zusammen (The Zame Zide!)
german entgegen (The Epposite side!)
N Goalby
1
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3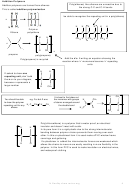 4
4