Histocompatibility & Immunogenetics
ADVERTISEMENT
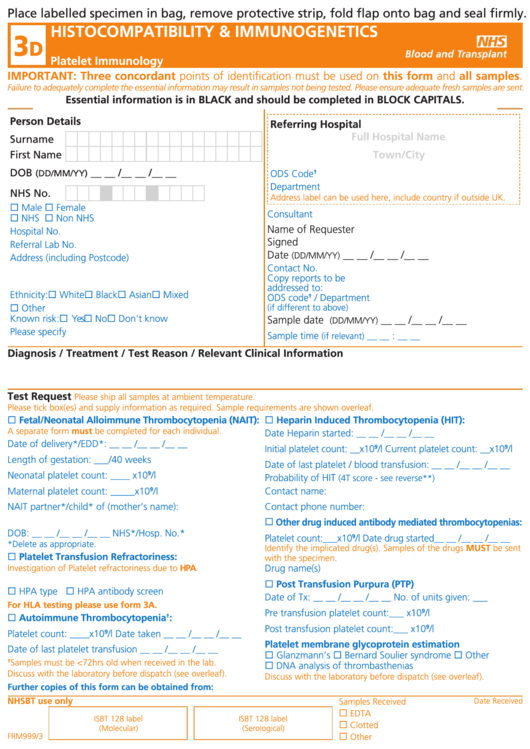
Place labelled specimen in bag, remove protective strip, fold flap onto bag and seal firmly.
HISTOCOMPATIBILITY & IMMUNOGENETICS
3
d
Platelet Immunology
IMPORTANT: Three concordant points of identification must be used on this form and all samples.
Failure to adequately complete the essential information may result in samples not being tested. Please ensure adequate fresh samples are sent.
Essential information is in BLACK and should be completed in BLOCK CAPITALS.
Person Details
Referring Hospital
Full Hospital Name
Surname
................................................................................
First Name
Town/City
................................................................................
DOB
__ __ /__ __ /__ __
ODS Code
......................................................................
(DD/MM/YY)
†
Department .....................................................................
NHS No.
Address label can be used here, include country if outside UK.
Male
Female
Consultant .......................................................................
NHS
Non NHS
Name of Requester ..................................................
Hospital No. ....................................................................
Signed .....................................................................
Referral Lab No. ..............................................................
Date
__ __ /__ __ /__ __
(DD/MM/YY)
Address (including Postcode) ............................................
Contact No. ....................................................................
........................................................................................
Copy reports to be
........................................................................................
addressed to: ..................................................................
Ethnicity: White Black Asian Mixed
ODS code
†
/ Department
..........................................................
Other ...........................................................................
(if different to above)
Known risk: Yes No Don't know
Sample date
__ __ /__ __ /__ __
(DD/MM/YY)
Please specify ...................................................................
Sample time
__ __ : __ __
(if relevant)
Diagnosis / Treatment / Test Reason / Relevant Clinical Information
Test Request
Please ship all samples at ambient temperature.
Please tick box(es) and supply information as required. Sample requirements are shown overleaf.
Fetal/Neonatal Alloimmune Thrombocytopenia (NAIT):
Heparin Induced Thrombocytopenia (HIT):
A separate form must be completed for each individual.
Date Heparin started: __ __ /__ __ /__ __
Date of delivery*/EDD*: __ __ /__ __ /__ __
Initial platelet count: __x10
/l Current platelet count: __x10
/l
9
9
Length of gestation: ___/40 weeks
Date of last platelet / blood transfusion: __ __ /__ __ /__ __
Neonatal platelet count: ____ x10
9
/l
Probability of HIT ...............................
(4T score - see reverse**)
Maternal platelet count: _____x10
9
/l
Contact name: .................................................................
NAIT partner*/child* of (mother’s name):
Contact phone number: ...................................................
........................................................................................
Other drug induced antibody mediated thrombocytopenias:
DOB: __ __ /__ __ /__ __ NHS*/Hosp. No.* ....................
Platelet count:___x10
/l Date drug started__ __ /__ __ /__ __
9
*Delete as appropriate.
Identify the implicated drug(s). Samples of the drugs MUST be sent
Platelet Transfusion Refractoriness:
with the specimen.
Investigation of Platelet refractoriness due to HPA.
Drug name(s) ..................................................................
N.B. HLA antibody investigation must be carried out first.
Post Transfusion Purpura (PTP)
HPA type
HPA antibody screen
Date of Tx: __ __ /__ __ /__ __ No. of units given: ___
For HLA testing please use form 3A.
Pre transfusion platelet count:___ x10
9
/l
Autoimmune Thrombocytopenia
:
†
Post transfusion platelet count:___ x10
/l
9
Platelet count: ____x10
9
/l Date taken __ __ /__ __ /__ __
Platelet membrane glycoprotein estimation
Date of last platelet transfusion __ __ /__ __ /__ __
Glanzmann’s Bernard Soulier syndrome Other
Samples must be <72hrs old when received in the lab.
†
DNA analysis of thrombasthenias
Discuss with the laboratory before dispatch (see overleaf).
Discuss with the laboratory before dispatch (see overleaf).
Further copies of this form can be obtained from:
NHSBT use only
Samples Received
Date Received
EDTA
ISBT 128 label
ISBT 128 label
Clotted
(Molecular)
(Serological)
FRM999/3
Other
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Life
 1
1 2
2