Lewis Structures And Resonance Worksheet
ADVERTISEMENT
Name: _______________________________________________________
Date:____________________________
Homework 7 – Lewis structures and resonance
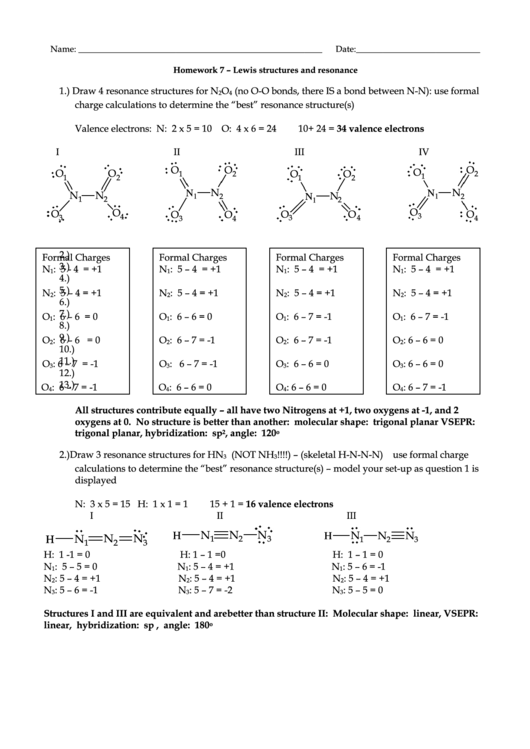
1.) Draw 4 resonance structures for N
O
(no O-O bonds, there IS a bond between N-N): use formal
2
4
charge calculations to determine the “best” resonance structure(s)
Valence electrons: N: 2 x 5 = 10 O: 4 x 6 = 24
10+ 24 = 34 valence electrons
I
II
III
IV
O
O
O
O
O
O
O
O
2
1
2
1
1
2
1
2
N
N
N
N
N
N
N
2
2
N
1
1
1
2
2
1
O
O
O
O
O
O
O
O
3
4
3
3
4
4
3
4
2.)
Formal Charges
Formal Charges
Formal Charges
Formal Charges
3.)
N
: 5 - 4 = +1
N
: 5 – 4 = +1
N
: 5 – 4 = +1
N
: 5 – 4 = +1
1
1
1
1
4.)
5.)
N
: 5 – 4 = +1
N
: 5 – 4 = +1
N
: 5 – 4 = +1
N
: 5 – 4 = +1
2
2
2
2
6.)
7.)
O
: 6 – 6 = 0
O
: 6 – 6 = 0
O
: 6 – 7 = -1
O
: 6 – 7 = -1
1
1
1
1
8.)
9.)
O
: 6 – 6 = 0
O
: 6 – 7 = -1
O
: 6 – 7 = -1
O
: 6 – 6 = 0
2
2
2
2
10.)
11.)
O
: 6 – 7 = -1
O
: 6 – 7 = -1
O
: 6 – 6 = 0
O
: 6 – 6 = 0
3
3
3
3
12.)
13.)
O
: 6 – 7 = -1
O
: 6 – 6 = 0
O
: 6 – 6 = 0
O
: 6 – 7 = -1
4
4
4
4
All structures contribute equally – all have two Nitrogens at +1, two oxygens at -1, and 2
oxygens at 0. No structure is better than another: molecular shape: trigonal planar VSEPR:
trigonal planar, hybridization: sp
, angle: 120
2
o
2.) Draw 3 resonance structures for HN
(NOT NH
!!!!) – (skeletal H-N-N-N) use formal charge
3
3
calculations to determine the “best” resonance structure(s) – model your set-up as question 1 is
displayed
N: 3 x 5 = 15 H: 1 x 1 = 1
15 + 1 = 16 valence electrons
I
II
III
N
N
N
N
N
N
N
N
N
H
H
1
2
3
1
2
3
H
1
2
3
H: 1 -1 = 0
H: 1 – 1 =0
H: 1 – 1 = 0
N
: 5 – 5 = 0
N
: 5 – 4 = +1
N
: 5 – 6 = -1
1
1
1
N
: 5 – 4 = +1
N
: 5 – 4 = +1
N
: 5 – 4 = +1
2
2
2
N
: 5 – 6 = -1
N
: 5 – 7 = -2
N
: 5 – 5 = 0
3
3
3
Structures I and III are equivalent and are better than structure II: Molecular shape: linear, VSEPR:
linear, hybridization: sp , angle: 180
o
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2