Chemistry: Counting Atoms In Compounds Worksheet
ADVERTISEMENT
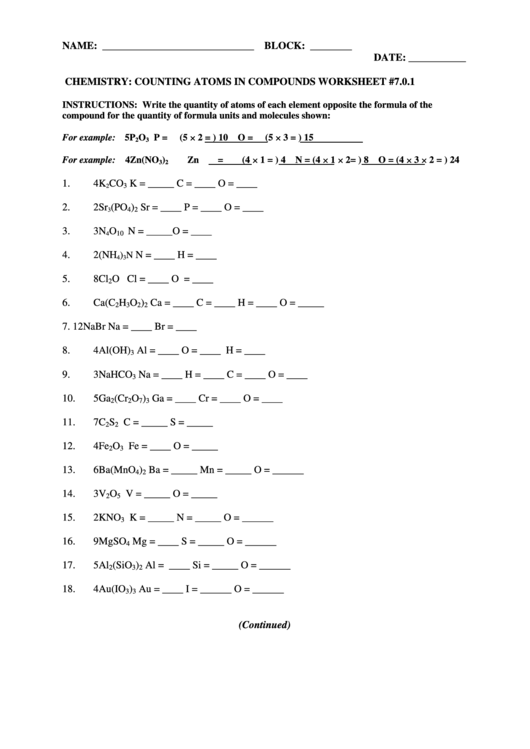
NAME: _____________________________
BLOCK: ________
DATE: ___________
CHEMISTRY: COUNTING ATOMS IN COMPOUNDS WORKSHEET #7.0.1
INSTRUCTIONS: Write the quantity of atoms of each element opposite the formula of the
compound for the quantity of formula units and molecules shown:
(5 H 2 = ) 10
(5 H 3 = ) 15
For example: 5P
O
P =
O =
2
3
Zn = (4 H 1 = ) 4 N = (4 H 1 H 2= ) 8 O = (4 H 3 H 2 = ) 24
For example: 4Zn(NO
)
3
2
1.
4K
CO
K = _____
C = ____
O = ____
3
2
2.
2Sr
(PO
)
Sr = ____
P = ____
O = ____
4
2
3
3.
3N
O
N = _____
O = ____
10
4
4.
2(NH
N = ____
H = ____
)
N
4
3
5.
8Cl
O
Cl = ____
O = ____
2
6.
Ca(C
H
O
)
Ca = ____
C = ____
H = ____
O = _____
2
3
2
2
7.
12NaBr
Na = ____
Br = ____
8.
4Al(OH)
Al = ____
O = ____
H = ____
3
9.
3NaHCO
Na = ____
H = ____
C = ____
O = ____
3
10.
5Ga
(Cr
O
)
Ga = ____
Cr = ____
O = ____
2
2
7
3
11.
7C
S
C = _____
S = _____
2
2
12.
4Fe
O
Fe = ____
O = _____
2
3
13.
6Ba(MnO
)
Ba = _____
Mn = _____ O = ______
4
2
14.
3V
O
V = _____
O = _____
2
5
15.
2KNO
K = _____
N = _____
O = ______
3
16.
9MgSO
Mg = ____
S = _____
O = ______
4
17.
5Al
(SiO
)
Al = ____
Si = _____
O = ______
2
3
2
18.
4Au(IO
)
Au = ____
I = ______
O = ______
3
3
(Continued)
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2








