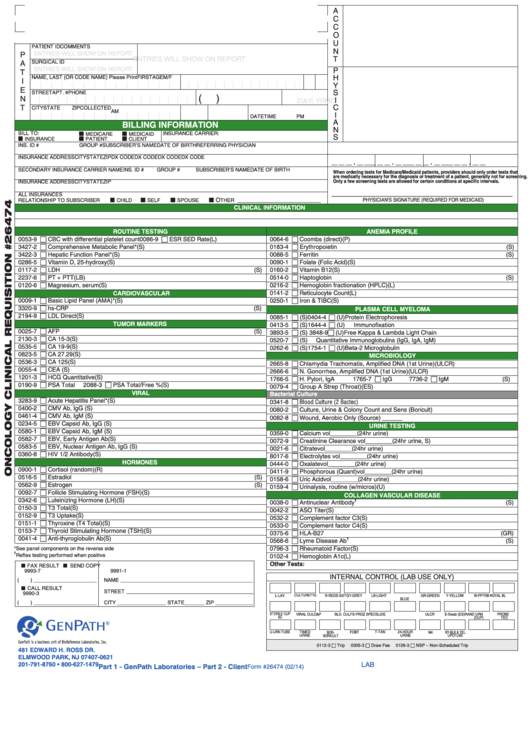
Form 26474 - Oncology Clinical Requisition
ADVERTISEMENT
A
C
C
O
U
PATIENT ID
COMMENTS
N
ENTRIES WILL SHOW ON REPORT
P
ENTRIES WILL SHOW ON REPORT
T
SURGICAL ID
A
ENTRIES WILL SHOW ON REPORT
P
T
H
NAME, LAST (OR CODE NAME) Please Print
FIRST
AGE M/F
I
Y
E
S
STREET
APT. # PHONE NO.
DATE OF BIRTH
(
)
N
I
MO
DAY YR
T
C
CITY
STATE
ZIP
COLLECTED
AM
I
DATE
TIME
PM
A
BILLING INFORMATION
N
BILL TO:
INSURANCE CARRIER:
I I
I I
MEDICARE
MEDICAID
S
I I
I I
I I
INSURANCE
PATIENT
CLIENT
INS. ID #
GROUP #
SUBSCRIBER’S NAME
DATE OF BIRTH
REFERRING PHYSICIAN
INSURANCE ADDRESS
CITY
STATE
ZIP
DX CODE
DX CODE
DX CODE
DX CODE
.
.
.
.
__ __ __
__ __
__ __ __
__ __
__ __ __
__ __
__ __ __
__ __
SECONDARY INSURANCE CARRIER NAME
INS. ID #
GROUP #
SUBSCRIBER’S NAME
DATE OF BIRTH
When ordering tests for Medicare/Medicaid patients, providers should only order tests that
are medically necessary for the diagnosis or treatment of a patient, generally not for screening.
INSURANCE ADDRESS
CITY
STATE
ZIP
Only a few screening tests are allowed for certain conditions at specific intervals.
___________________________________________
ALL INSURANCES
I I
I I
I I
I I
O
PHYSICIAN’S SIGNATURE (REQUIRED FOR MEDICAID)
RELATIONSHIP TO SUBSCRIBER
CHILD
SELF
SPOUSE
THER _____________________________
CLINICAL INFORMATION
ROUTINE TESTING
ANEMIA PROFILE
0053-9
CBC with differential platelet count
0086-9
ESR SED Rate
(L)
0064-6
Coombs (direct)
(P)
3427-2
Comprehensive Metabolic Panel*
(S)
0183-4
Erythropoietin
(S)
3422-3
Hepatic Function Panel*
(S)
0088-5
Ferritin
(S)
0286-5
Vitamin D, 25-hydroxy
(S)
0090-1
Folate (Folic Acid)
(S)
0117-2
LDH
(S)
0160-2
Vitamin B12
(S)
2237-6
PT + PTT
(LB)
0514-0
Haptoglobin
(S)
0120-6
Magnesium, serum
(S)
0216-2
Hemoglobin fractionation (HPLC)
(L)
CARDIOVASCULAR
0141-2
Reticulocyte Count
(L)
0009-1
Basic Lipid Panel (AMA)*
(S)
0250-1
Iron & TIBC
(S)
3320-9
hs-CRP
(S)
PLASMA CELL MYELOMA
2194-9
LDL Direct
(S)
0085-1
(S)
0404-4
(U)
Protein Electrophoresis
TUMOR MARKERS
0413-5
(S)
1644-4
(U)
Immunofixation
0025-7
AFP
(S)
3893-5
(S)
3848-9
(U)
Free Kappa & Lambda Light Chain
2130-3
CA 15-3
(S)
0520-7
(S)
Quantitative Immunoglobulins (IgG, IgA, IgM)
0535-5
CA 19-9
(S)
0262-6
(S)
1754-1
(U)
Beta-2 Microglobulin
0823-5
CA 27.29
(S)
MICROBIOLOGY
0536-3
CA 125
(S)
2665-8
Chlamydia Trachomatis, Amplified DNA (1st Urine)
(ULCR)
0055-4
CEA
(S)
2666-6
N. Gonorrhea, Amplified DNA (1st Urine)
(ULCR)
1201-3
HCG Quantitative
(S)
1766-5
H. Pylori, IgA
1765-7
IgG
7736-2
IgM
(S)
0190-9
PSA Total
2088-3
PSA Total/Free %
(S)
0079-4
Group A Strep (Throat)
(ES)
VIRAL
Bacterial Culture
3283-9
Acute Hepatitis Panel*
(S)
0341-8
Blood Culture (2 Bactec)
0400-2
CMV Ab, IgG
(S)
0080-2
Culture, Urine & Colony Count and Sens (Boricult)
0461-4
CMV Ab, IgM
(S)
0082-8
Wound, Aerobic Only (Source) ______
0234-5
EBV Capsid Ab, IgG
(S)
URINE TESTING
0580-1
EBV Capsid Ab, IgM
(S)
0359-0
Calcium
vol________
(24hr urine)
0582-7
EBV, Early Antigen Ab
(S)
0072-9
Creatinine Clearance
vol________
(24hr urine, S)
0583-5
EBV, Nuclear Antigen Ab, IgG
(S)
0021-6
Citrate
vol________
(24hr urine)
0360-8
HIV 1/2 Antibody
(S)
8017-6
Electrolytes
vol________
(24hr urine)
HORMONES
0444-0
Oxalate
vol________
(24hr urine)
0900-1
Cortisol (random)
(R)
0411-9
Phosphorous (Quant)
vol________
(24hr urine)
0516-5
Estradiol
(S)
0158-6
Uric Acid
vol________
(24hr urine)
0562-9
Estrogen
(S)
0159-4
Urinalysis, routine (w/micros)
(U)
0092-7
Follicle Stimulating Hormone (FSH)
(S)
COLLAGEN VASCULAR DISEASE
0342-6
Luteinizing Hormone (LH)
(S)
1
0038-0
Antinuclear Antibody
(S)
0150-3
T3 Total
(S)
0042-2
ASO Titer
(S)
0152-9
T3 Uptake
(S)
0532-2
Complement factor C3
(S)
0151-1
Thyroxine (T4 Total)
(S)
0533-0
Complement factor C4
(S)
0153-7
Thyroid Stimulating Hormone (TSH)
(S)
0375-6
HLA-B27
(GR)
0041-4
Anti-thyroglobulin Ab
(S)
1
0568-6
Lyme Disease Ab
(S)
*See panel components on the reverse side
0796-3
Rheumatoid Factor
(S)
1
Reflex testing performed when positive
0102-4
Hemoglobin A1c
(L)
Other Tests:
I I
FAX RESULT
I I
SEND COPY
9993-7
9991-1
INTERNAL CONTROL (LAB USE ONLY)
(
) ______________________
NAME ______________________________________________
I I
CALL RESULT
STREET ____________________________________________
9990-3
L-LAV
CULTURETTE
R-RED
S-SST
GY-GREY
LB-LIGHT
GR-GREEN
Y-YELLOW
W-PPT
RB-ROYAL BL
BLUE
CITY _________________ STATE_______ ZIP _____________
(
) ______________________
STERILE CUP
VIRAL CUL
O&P
BLD. CUL
FS-FROZ SPEC
SLIDE
ULCR
E-Swab (ES)
RAND URN
PROBE
SC
(CUP)
TEC
U-URN TUBE
TIMED
T-TAN
24-HOUR
BOR-
FOBT
M4
BY-BLK & YEL
URINE
BORICULT
URINE
LIPOTUBE
GenPath is a business unit of BioReference Laboratories, Inc.
0112-3
Trip
0305-3
Draw Fee
0126-3
NSP – Non-Scheduled Trip
481 EDWARD H. ROSS DR.
ELMWOOD PARK, NJ 07407-0621
201-791-8760 • 800-627-1479
LAB I.D. NO.
Part 1 - GenPath Laboratories – Part 2 - Client
Form #26474 (02/14)
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Business
 1
1 2
2








