Solutions Sheet For Weak Acids Or Weak Bases
ADVERTISEMENT
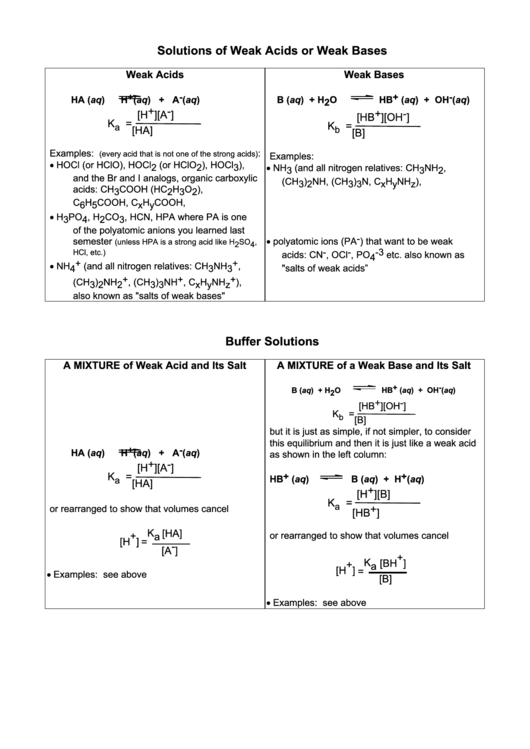
Solutions of Weak Acids or Weak Bases
Weak Acids
Weak Bases
H + (aq) + A - (aq)
HB + (aq) + OH - (aq)
HA (aq)
B (aq) + H 2 O
[H + ] [A - ]
[HB + ] [OH - ]
K
=
K
=
a
[HA]
b
[B]
Examples:
:
(every acid that is not one of the strong acids)
Examples:
• HOCl (or HClO), HOCl 2 (or HClO 2 ), HOCl 3 ),
•
NH 3 (and all nitrogen relatives: CH 3 NH 2 ,
and the Br and I analogs, organic carboxylic
(CH 3 ) 2 NH, (CH 3 ) 3 N, C x H y NH z ),
acids: CH 3 COOH (HC 2 H 3 O 2 ),
C 6 H 5 COOH, C x H y COOH,
• H 3 PO 4 , H 2 CO 3 , HCN, HPA where PA is one
of the polyatomic anions you learned last
polyatomic ions (PA - ) that want to be weak
•
semester
(unless HPA is a strong acid like H 2 SO 4 ,
acids: CN - , OCl - , PO 4 -3 etc. also known as
HCl, etc.)
• NH 4 + (and all nitrogen relatives: CH 3 NH 3 + ,
"salts of weak acids”
(CH 3 ) 2 NH 2 + , (CH 3 ) 3 NH + , C x H y NH z + ),
also known as "salts of weak bases"
Buffer Solutions
A MIXTURE of Weak Acid and Its Salt
A MIXTURE of a Weak Base and Its Salt
HB + (aq) + OH - (aq)
B (aq) + H 2 O
[HB + ] [OH - ]
K
=
b
[B]
but it is just as simple, if not simpler, to consider
this equilibrium and then it is just like a weak acid
H + (aq) + A - (aq)
HA (aq)
as shown in the left column:
[H + ] [A - ]
K
=
B (aq) + H + (aq)
HB + (aq)
a
[HA]
[H + ] [B]
K
=
a
[HB + ]
or rearranged to show that volumes cancel
K a [HA]
or rearranged to show that volumes cancel
+
[H
] =
[A - ]
K a [BH + ]
+
[H
] =
•
Examples: see above
[B]
•
Examples: see above
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2








