Form Wc-51 - Special Formula Request Form - Arkansas Department Of Health
ADVERTISEMENT
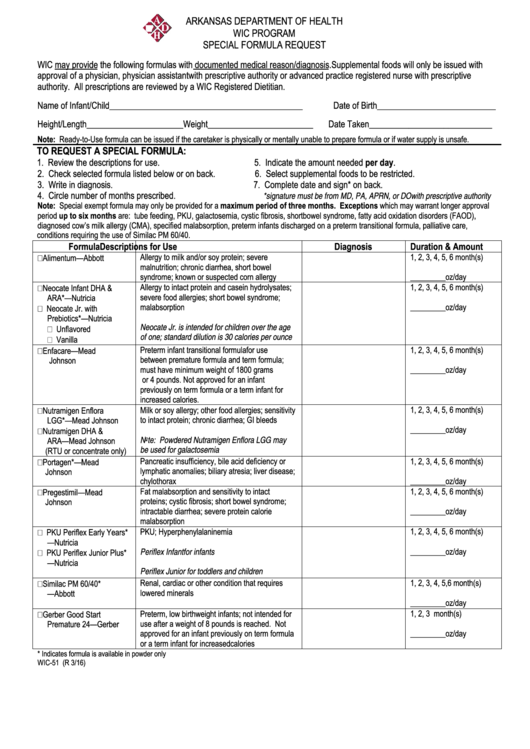
ARKANSAS DEPARTMENT OF HEALTH
WIC PROGRAM
SPECIAL FORMULA REQUEST
WIC may provide the following formulas with documented medical reason/diagnosis. Supplemental foods will only be issued with
approval of a physician, physician assistant with prescriptive authority or advanced practice registered nurse with prescriptive
authority. All prescriptions are reviewed by a WIC Registered Dietitian.
Name of Infant/Child____________________________________________
Date of Birth___________________________
Height/Length______________________ Weight________________________
Date Taken____________________________
Note: Ready-to-Use formula can be issued if the caretaker is physically or mentally unable to prepare formula or if water supply is unsafe.
TO REQUEST A SPECIAL FORMULA:
5. Indicate the amount needed per day.
1. Review the descriptions for use.
2. Check selected formula listed below or on back.
6. Select supplemental foods to be restricted.
3. Write in diagnosis.
7. Complete date and sign* on back.
4. Circle number of months prescribed.
*signature must be from MD, PA, APRN, or DO with prescriptive authority
Note: Special exempt formula may only be provided for a maximum period of three months. Exceptions which may warrant longer approval
period up to six months are: tube feeding, PKU, galactosemia, cystic fibrosis, short bowel syndrome, fatty acid oxidation disorders (FAOD),
diagnosed cow’s milk allergy (CMA), specified malabsorption, preterm infants discharged on a preterm transitional formula, palliative care,
conditions requiring the use of Similac PM 60/40.
Formula
Descriptions for Use
Diagnosis
Duration & Amount
Allergy to milk and/or soy protein; severe
1, 2, 3, 4, 5, 6 month(s)
Alimentum—Abbott
malnutrition; chronic diarrhea, short bowel
syndrome; known or suspected corn allergy
_________oz/day
Allergy to intact protein and casein hydrolysates;
1, 2, 3, 4, 5, 6 month(s)
Neocate Infant DHA &
severe food allergies; short bowel syndrome;
ARA*—Nutricia
malabsorption
_________oz/day
Neocate Jr. with
Prebiotics*—Nutricia
Neocate Jr. is intended for children over the age
Unflavored
of one; standard dilution is 30 calories per ounce
Vanilla
Preterm infant transitional formula for use
1, 2, 3, 4, 5, 6 month(s)
Enfacare—Mead
between premature formula and term formula;
Johnson
must have minimum weight of 1800 grams
_________oz/day
or 4 pounds. Not approved for an infant
previously on term formula or a term infant for
increased calories.
Milk or soy allergy; other food allergies; sensitivity
1, 2, 3, 4, 5, 6 month(s)
Nutramigen Enflora
to intact protein; chronic diarrhea; GI bleeds
LGG*—Mead Johnson
_________oz/day
Nutramigen DHA &
Note: Powdered Nutramigen Enflora LGG may
ARA—Mead Johnson
be used for galactosemia
(RTU or concentrate only)
Pancreatic insufficiency, bile acid deficiency or
1, 2, 3, 4, 5, 6 month(s)
Portagen*—Mead
lymphatic anomalies; biliary atresia; liver disease;
Johnson
chylothorax
_________oz/day
Fat malabsorption and sensitivity to intact
1, 2, 3, 4, 5, 6 month(s)
Pregestimil—Mead
proteins; cystic fibrosis; short bowel syndrome;
Johnson
intractable diarrhea; severe protein calorie
_________oz/day
malabsorption
PKU; Hyperphenylalaninemia
1, 2, 3, 4, 5, 6 month(s)
PKU Periflex Early Years*
—Nutricia
_________oz/day
Periflex Infant for infants
PKU Periflex Junior Plus*
—Nutricia
Periflex Junior for toddlers and children
Renal, cardiac or other condition that requires
1, 2, 3, 4, 5, 6 month(s)
Similac PM 60/40*
lowered minerals
—Abbott
_________oz/day
Preterm, low birthweight infants; not intended for
1, 2, 3 month(s)
Gerber Good Start
use after a weight of 8 pounds is reached. Not
Premature 24—Gerber
approved for an infant previously on term formula
_________oz/day
or a term infant for increased calories
* Indicates formula is available in powder only
WIC-51 (R 3/16)
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Medical
 1
1 2
2








