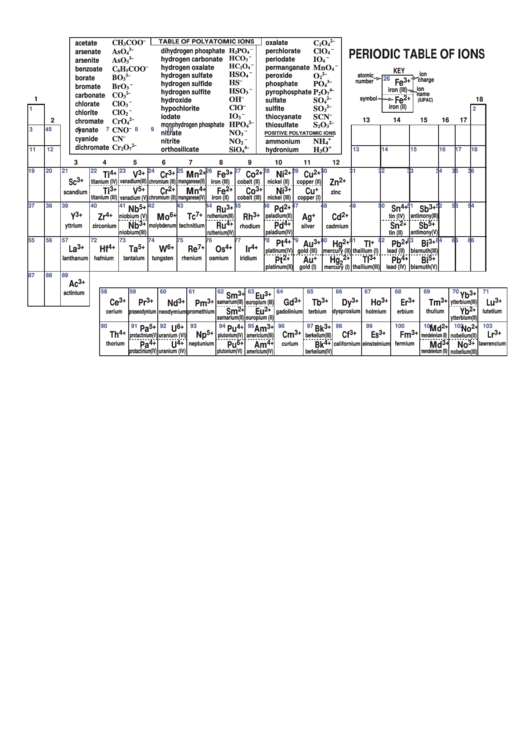
Periodic Table Of Ions
ADVERTISEMENT
–
TABLE OF POLYATOMIC IONS
oxalate
2–
acetate
CH
COO
C
O
3
2
4
–
–
3–
dihydrogen phosphate
perchlorate
arsenate
H
PO
ClO
PERIODIC TABLE OF IONS
AsO
2
4
4
4
–
–
3–
hydrogen carbonate
HCO
periodate
IO
arsenite
AsO
3
4
3
–
hydrogen oxalate
HC
O
permanganate
–
–
MnO
benzoate
C
H
COO
2
4
4
KEY
6
5
–
ion
HSO
2–
hydrogen sulfate
peroxide
atomic
O
3–
borate
4
BO
2
26
charge
3+
3
number
Fe
–
HS
3–
hydrogen sulfide
phosphate
PO
–
bromate
BrO
4
ion
3
iron (III)
–
HSO
4–
hydrogen sulfite
pyrophosphate
P
O
2–
3
name
carbonate
CO
2
7
3
2+
–
symbol
1
Fe
OH
2–
hydroxide
sulfate
18
SO
(IUPAC)
–
4
chlorate
ClO
iron (II)
3
–
ClO
2–
hypochlorite
sulfite
SO
1
2
–
3
chlorite
ClO
–
2
–
iodate
IO
thiocyanate
SCN
3
2–
13
14
15
16
17
2
chromate
CrO
2–
4
2–
monohydrogen phosphate
HPO
thiosulfate
S
O
4
2
3
–
cyanate
5
6
7
8
9
10
3
4
CNO
–
nitrate
NO
POSITIVE POLYATOMIC IONS
3
–
cyanide
CN
+
–
nitrite
NO
ammonium
NH
2
4
2–
dichromate
Cr
O
+
4–
orthosilicate
SiO
hydronium
H
O
11
12
2
7
13
14
15
16
17
18
4
3
3
4
5
6
7
8
9
10
11
12
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
2+
3+
2+
2+
2+
4+
3+
3+
Ti
V
Cr
Mn
Fe
Co
Ni
Cu
3+
2+
vanadium(III)
manganese(II)
Sc
titanium (IV)
chromium (III)
iron (III)
cobalt (II)
nickel (II)
copper (II)
Zn
3+
5+
2+
4+
2+
3+
3+
+
Ti
V
Cr
Mn
Fe
Co
Ni
Cu
scandium
zinc
titanium (III)
chromium (II)
manganese(IV)
iron (II)
cobalt (III)
nickel (III)
copper (I)
vanadium (V)
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
5+
2+
4+
3+
3+
Sn
Nb
Ru
Pd
Sb
3+
4+
6+
7+
3+
+
2+
Y
Zr
Mo
Tc
Rh
paladium(II)
Ag
Cd
antimony(III)
niobium (V)
ruthenium(III)
tin (IV)
3+
4+
4+
2+
5+
Nb
Ru
Pd
Sn
Sb
yttrium
molybdenum
technitium
zirconium
rhodium
silver
cadmium
paladium(IV)
antimony(V)
niobium(III)
ruthenium(IV)
tin (II)
55
56
57
72
73
74
75
76
77
78
79
80
81
82
83
84
85
86
4+
3+
3+
2+
+
2+
Pt
Bi
Au
Hg
Tl
Pb
3+
4+
5+
6+
7+
4+
4+
La
Hf
Ta
W
Re
Os
Ir
platinum(IV)
gold (III)
mercury (II)
thallium (I)
lead (II)
bismuth(III)
+
2+
3+
lanthanum
hafnium
tantalum
tungsten
rhenium
osmium
iridium
2+
4+
5+
Pt
Au
Hg
Tl
Pb
Bi
2
platinum(II)
gold (I)
mercury (I)
thallium(III)
lead (IV)
bismuth(V)
87
88
89
3+
Ac
58
59
60
61
62
63
64
65
66
67
68
69
70
71
actinium
3+
3+
3+
Yb
Sm
Eu
3+
3+
3+
3+
3+
3+
3+
3+
3+
3+
3+
Ce
Pr
Gd
Tb
Dy
Ho
Er
Tm
Lu
Nd
Pm
samarium(III)
europium (III)
ytterbium(III)
2+
2+
2+
Sm
Eu
Yb
cerium
praseodymium
neodymium
promethium
gadolinium
terbium
dysprosium
holmium
erbium
thulium
lutetium
samarium(II)
europium (II)
ytterbium(II)
90
91
5+
92
6+
93
94
4+
95
3+
96
97
3+
98
99
100
101
2+
102
2+
103
Pa
U
Pu
Am
Bk
Md
No
4+
5+
3+
3+
3+
3+
3+
Th
Np
Cm
Cf
Es
Fm
Lr
protactinium(V)
uranium (VI)
plutonium(IV)
americium(III)
berkelium(III)
mendelevium (II)
nobelium(II)
4+
4+
6+
4+
4+
3+
3+
thorium
neptunium
curium
californium
einsteinium
fermium
lawrencium
Pa
U
Pu
Am
Bk
Md
No
protactinium(IV)
uranium (IV)
plutonium(VI)
americium(IV)
berkelium(IV)
mendelevium (III)
nobelium(III)
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1








