Vsepr Theory (Molecular Shapes)
ADVERTISEMENT
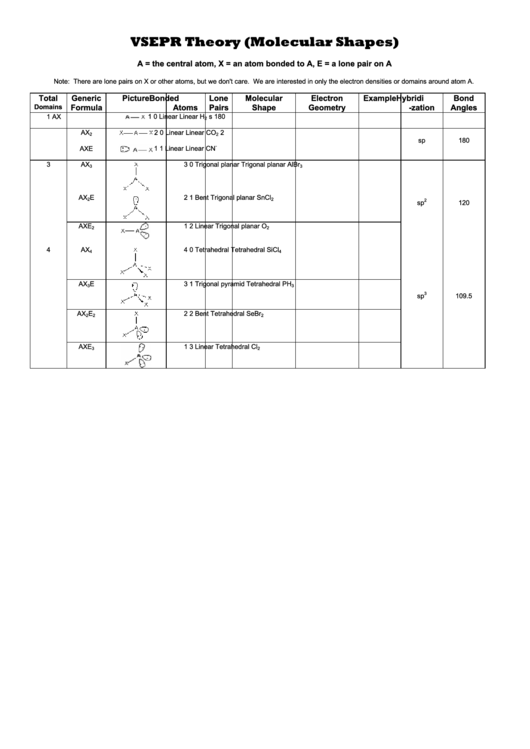
VSEPR Theory (Molecular Shapes)
A = the central atom, X = an atom bonded to A, E = a lone pair on A
Note: There are lone pairs on X or other atoms, but we don't care. We are interested in only the electron densities or domains around atom A.
Total
Generic
Picture
Bonded
Lone
Molecular
Electron
Example
Hybridi
Bond
Domains
Formula
Atoms
Pairs
Shape
Geometry
-zation
Angles
1
AX
1
0
Linear
Linear
H
s
180
2
2
AX
2
0
Linear
Linear
CO
2
2
sp
180
-
AXE
1
1
Linear
Linear
CN
3
AX
3
0
Trigonal planar
Trigonal planar
AlBr
3
3
AX
E
2
1
Bent
Trigonal planar
SnCl
2
2
2
sp
120
AXE
1
2
Linear
Trigonal planar
O
2
2
4
AX
4
0
Tetrahedral
Tetrahedral
SiCl
4
4
AX
E
3
1
Trigonal pyramid
Tetrahedral
PH
3
3
3
sp
109.5
AX
E
2
2
Bent
Tetrahedral
SeBr
2
2
2
AXE
1
3
Linear
Tetrahedral
Cl
3
2
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2