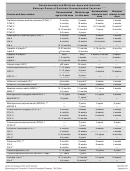
Recommended And Minimum Ages And Intervals Between Doses Of Routinely Recommended Vaccines Page 2
ADVERTISEMENT
1 Combination vaccines are available. Use of licensed combination vaccines is generally preferred to separate injections of their
equivalent component vaccines. When administering combination vaccines, the minimum age for administration is the oldest age
for any of the individual components. The minimum interval between doses is equal to the greatest interval of any of the individual
components.
2 Information on travel vaccines including typhoid, Japanese encephalitis, and yellow fever, is available at
Information on other vaccines that are licensed in the US but not distributed, including anthrax and smallpox, is available at
https://emergency.cdc.gov/bioterrorism/.
3 “Months” refers to calendar months.
4 A hyphen used to express a range (as in “12-15 months”) means “through.”
5 Combination vaccines containing a hepatitis B component (Pediarix and Twinrix) are available. These vaccines should not be
administered to infants younger than 6 weeks because of the other components (i.e., Hib, DTaP, HepA, and IPV).
6 The minimum recommended interval between DTaP-3 and DTaP-4 is 6 months. However, DTaP-4 need not be repeated if
administered at least 4 months after DTaP-3. This is a special grace period of 2 months, which can be used when evaluating
records retrospectively. An additional 4 days should not be added to this grace period prospectively, but can be added
retrospectively.
7 If a fourth dose of DTaP is given on or after the fourth birthday, a fifth dose is not needed.
8 Children receiving the first dose of Hib or PCV13 vaccine at age 7 months or older require fewer doses to complete the series.
9 If PedvaxHib is administered at ages 2 and 4 months, a dose at age 6 months is not required. The minimum age for the final
dose is 12 months.
10 HepB-3 should be administered at least 8 weeks after HepB-2 and at least 16 weeks after HepB-1, and should not be
administered before 24 weeks of age.
11 Herpes zoster vaccine is recommended as a single dose for persons 60 years of age and older.
12 Gardasil and Gardasil 9 are approved for males and females 9 through 26 years of age. The minimum age for HPV-3 is
based on the baseline minimum age for the first dose (i.e., 9 years) and the minimum interval of 5 months between the first
and third dose. Dose 3 need not be repeated if it is administered at least 5 months after the first dose, and if the intervals
between doses 1 and 2, and doses 2 and 3, are 4 weeks and 12 weeks, respectively.
th
13 A two-dose HPV vaccine schedule is recommended for most persons who begin the series before the 15
birthday. See
for details.
14 One dose of influenza vaccine per season is recommended for most people. Some children younger than 9 years of age
should receive 2 doses in a single season. See current influenza recommendations for details.
15 The minimum age for inactivated influenza vaccine varies by vaccine manufacturer. See package inserts for vaccine-specific
minimum ages.
16 Combination MMRV vaccine can be used for children 12 months through 12 years of age. See
for details.
17 Revaccination with meningococcal vaccine is recommended for previously vaccinated persons who remain at high risk for
meningococcal disease. See for details.
18 High-risk children can receive Menactra as young as 9 months and Menveo as young as 2 months. MenHibrix is given as a four-dose
series at 2, 4, 6, and 12-18 months. It can be given as young as 6 weeks for high-risk children.
19 For routine, non-high risk adolescent vaccination, the minimum age for the booster dose is 16 years.
20 A second dose of PPSV23 5 years after the first dose is recommended for persons <65 years of age at highest risk for serious
pneumococcal infection, and for those who are likely to have a rapid decline in pneumococcal antibody concentration. See
for details.
th
21 A fourth dose is not needed if the third dose was administered on or after the 4
birthday and at least 6 months after the
previous dose.
22 The first dose of rotavirus must be administered no earlier than 6 weeks and no later than 14 weeks 6 days. The vaccine series
should not be started for infants 15 weeks 0 days or older. Rotavirus vaccine should not be administered to children older than 8
months 0 days, regardless of the number of doses received before that age. If two doses of Rotarix are administered as age
appropriate, a third dose is not necessary.
23 Only one dose of Tdap is recommended. Subsequent doses should be given as Td. For management of a tetanus-prone wound
in a person who has received a primary series of a tetanus-toxoid containing vaccine, the minimum interval after a previous dose
of any tetanus-containing vaccine is 5 years.
24 A special grace period of 2 months, based on expert opinion, can be applied to the minimum interval of 3 months, when evaluating
records retrospectively, which results in an acceptable minimum interval of 4 weeks. An additional 4 days should not be added to
this grace period.
25 A special grace period of 2 months, based on expert opinion, can be applied to the minimum age of 15 months when evaluating
records retrospectively, which will result in an acceptable minimum age of 13 months. An additional 4 days should not be added to
this grace period.
Adapted from Table 3-1, ACIP General Best Practice Guidelines for Immunization.
September 2017
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Business
 1
1 2
2








