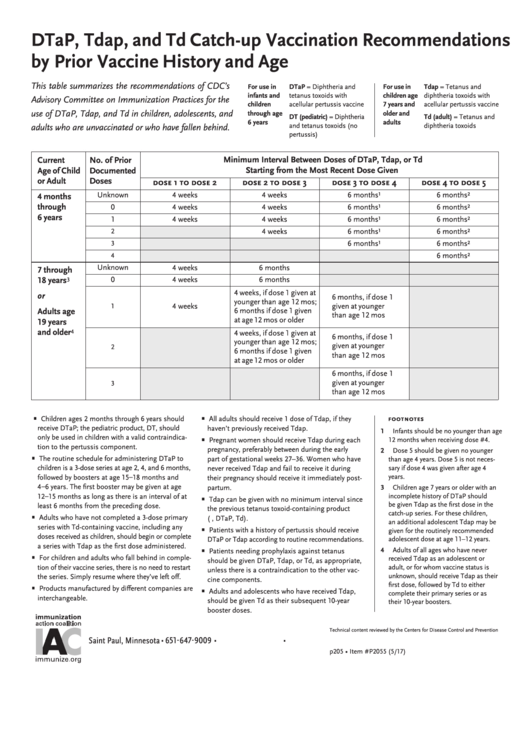
Dtap, Tdap, And Td Catch-Up Vaccination Recommendations By Prior Vaccine History And Age
ADVERTISEMENT
DTaP, Tdap, and Td Catch-up Vaccination Recommendations
by Prior Vaccine History and Age
This table summarizes the recommendations of CDC’s
For use in
DTaP = Diphtheria and
For use in
Tdap = Tetanus and
infants and
tetanus toxoids with
children age
diphtheria toxoids with
Advisory Committee on Immunization Practices for the
children
acellular pertussis vaccine
7 years and
acellular pertussis vaccine
use of DTaP, Tdap, and Td in children, adolescents, and
through age
older and
DT (pediatric) = Diphtheria
Td (adult) = Tetanus and
6 years
adults
and tetanus toxoids (no
diphtheria toxoids
adults who are unvaccinated or who have fallen behind.
pertussis)
Minimum Interval Between Doses of DTaP, Tdap, or Td
Current
No. of Prior
Starting from the Most Recent Dose Given
Age of Child
Documented
or Adult
Doses
dose 1 to dose 2
dose 2 to dose 3
dose 3 to dose 4
dose 4 to dose 5
Unknown
4 weeks
4 weeks
6 months
6 months
¹
²
4 months
through
0
4 weeks
4 weeks
6 months
6 months
¹
²
6 years
1
4 weeks
4 weeks
6 months
6 months
¹
²
4 weeks
6 months
6 months
2
¹
²
6 months
6 months
3
¹
²
4
6 months
²
Unknown
4 weeks
6 months
7 through
18 years
0
4 weeks
6 months
3
4 weeks, if dose 1 given at
or
6 months, if dose 1
younger than age 12 mos;
4 weeks
given at younger
1
Adults age
6 months if dose 1 given
than age 12 mos
at age 12 mos or older
19 years
and older
4
4 weeks, if dose 1 given at
6 months, if dose 1
younger than age 12 mos;
given at younger
2
6 months if dose 1 given
than age 12 mos
at age 12 mos or older
6 months, if dose 1
given at younger
3
than age 12 mos
• Children ages 2 months through 6 years should
• All adults should receive 1 dose of Tdap, if they
footnotes
receive DTaP; the pediatric product, DT, should
haven’t previously received Tdap.
1 Infants should be no younger than age
only be used in children with a valid contraindica-
• Pregnant women should receive Tdap during each
12 months when receiving dose #4.
tion to the pertussis component.
pregnancy, preferably between during the early
2 Dose 5 should be given no younger
• The routine schedule for administering DTaP to
part of gestational weeks 27–36. Women who have
than age 4 years. Dose 5 is not neces-
children is a 3-dose series at age 2, 4, and 6 months,
sary if dose 4 was given after age 4
never received Tdap and fail to receive it during
followed by boosters at age 15 – 18 months and
years.
their pregnancy should receive it immediately post-
4 – 6 years. The first booster may be given at age
partum.
3 Children age 7 years or older with an
12 – 15 months as long as there is an interval of at
incomplete history of DTaP should
• Tdap can be given with no minimum interval since
be given Tdap as the first dose in the
least 6 months from the preceding dose.
the previous tetanus toxoid-containing product
catch-up series. For these children,
• Adults who have not completed a 3-dose primary
(e.g., DTaP, Td).
an additional adolescent Tdap may be
series with Td-containing vaccine, including any
• Patients with a history of pertussis should receive
given for the routinely recommended
doses received as children, should begin or complete
DTaP or Tdap according to routine recommendations.
adolescent dose at age 11–12 years.
a series with Tdap as the first dose administered.
• Patients needing prophylaxis against tetanus
4 Adults of all ages who have never
• For children and adults who fall behind in comple-
received Tdap as an adolescent or
should be given DTaP, Tdap, or Td, as appropriate,
tion of their vaccine series, there is no need to restart
adult, or for whom vaccine status is
unless there is a contraindication to the other vac-
unknown, should receive Tdap as their
the series. Simply resume where they’ve left off.
cine components.
first dose, followed by Td to either
• Products manufactured by different companies are
• Adults and adolescents who have received Tdap,
complete their primary series or as
interchangeable.
should be given Td as their subsequent 10-year
their 10-year boosters.
booster doses.
Technical content reviewed by the Centers for Disease Control and Prevention
651 - 647 - 9009
Saint Paul, Minnesota
•
•
•
/catg.d/p2055.pdf
Item #P2055 (5/17)
•
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Medical
 1
1








