Chemistry Cookie Project Chocolate Chip Worksheet
ADVERTISEMENT
Chemistry Cookie Project –Chocolate Chip
• In this lab you will be converting a recipe from moles to standard cooking measurements
and then using that recipe to bake some cookies!
• You will need the following tables in order to convert your recipe:
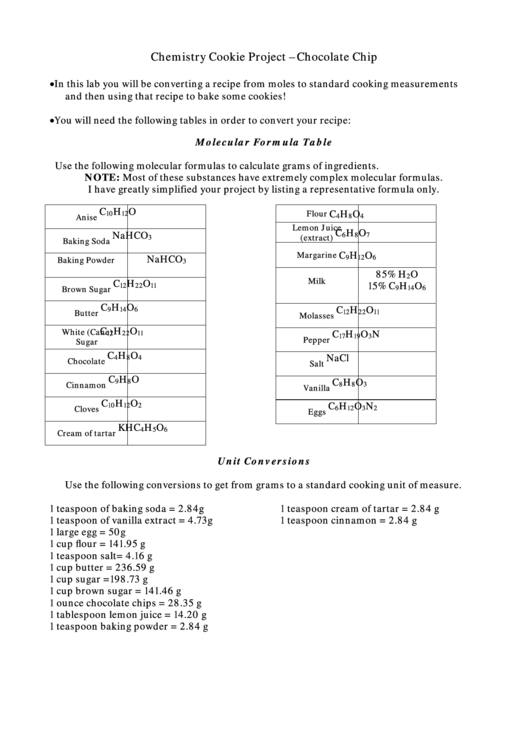
Molecular Formula Table
Use the following molecular formulas to calculate grams of ingredients.
NOTE: Most of these substances have extremely complex molecular formulas.
I have greatly simplified your project by listing a representative formula only.
C
H
O
C
H
O
Flour
10
12
Anise
4
8
4
Lemon Juice
C
H
O
NaHCO
6
8
7
(extract)
3
Baking Soda
C
H
O
Margarine
NaHCO
9
12
6
Baking Powder
3
85% H
O
2
Milk
C
H
O
15% C
H
O
12
22
11
Brown Sugar
9
14
6
C
H
O
C
H
O
9
14
6
Butter
12
22
11
Molasses
C
H
O
White (Cane)
C
H
O
N
12
22
11
17
19
3
Pepper
Sugar
C
H
O
NaCl
4
8
4
Chocolate
Salt
C
H
O
C
H
O
9
8
Cinnamon
8
8
3
Vanilla
C
H
O
C
H
O
N
10
12
2
Cloves
6
12
3
2
Eggs
KHC
H
O
4
5
6
Cream of tartar
Unit Conversions
Use the following conversions to get from grams to a standard cooking unit of measure.
1 teaspoon of baking soda = 2.84g
1 teaspoon cream of tartar = 2.84 g
1 teaspoon of vanilla extract = 4.73g
1 teaspoon cinnamon = 2.84 g
1 large egg = 50g
1 cup flour = 141.95 g
1 teaspoon salt= 4.16 g
1 cup butter = 236.59 g
1 cup sugar =198.73 g
1 cup brown sugar = 141.46 g
1 ounce chocolate chips = 28.35 g
1 tablespoon lemon juice = 14.20 g
1 teaspoon baking powder = 2.84 g
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3








