Acid-Base Equilibria And Buffer Solutions Worksheet With Answers
ADVERTISEMENT
Chem 1B
Chapter 15 Exercises
Exercise #1: Acid-Base Equilibria and Buffer Solutions
1.
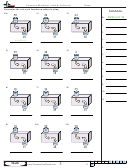
Consider the ionization equilibrium of acetic acid in aqueous solution:
⇌
–
+
CH
COOH
+ H
O
H
O
+ CH
CO
(aq)
(l)
(aq)
(aq)
3
2
3
3
2
+
(a) Does the equilibrium concentration of H
O
increase, decrease, or stays the same if some sodium
3
acetate, NaCH
CO
, is added to the acetic acid solution? Explain.
3
2
(b) Does the pH of the solution increase, decrease, or stay the same if some sodium acetate, NaCH
CO
,
3
2
is added to the acetic acid solution? Explain.
–
(c) Does the equilibrium concentration of acetate ion, CH
CO
, increase, decrease, or stay the same if
3
2
some sodium hydroxide is added to the solution? Explain. How will the pH change?
–
5
2.
Given that K
= 1.8 x 10
for acetic acid, calculate the pH of each of the following solutions:
a
(a) A solution containing 0.10 M acetic acid (CH
COOH) and 0.10 M sodium acetate (NaCH
CO
).
3
3
2
(b) A solution containing 0.10 M CH
CO
H and 0.050 M NaCH
CO
.
3
2
3
2
(c) A solution containing 0.050 M CH
CO
H and 0.10 M NaCH
CO
.
3
2
3
2
(Answer: (a) pH = 4.74; (b) pH = 4.44; (c) pH = 5.04)
3.
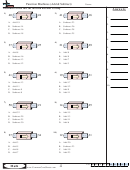
Consider the ionization equilibrium of ammonia in aqueous solution.
⇌
–
+
NH
+ H
O
NH
+ OH
(aq)
(l)
(aq)
(aq)
3
2
4
-
(a) Does the equilibrium concentration of OH
increase, decrease, or stay the same if ammonium
chloride (NH
Cl) is added to the ammonia solution? Explain.
4
(b) Does the pH of the pH of the solution increase, decrease, or stay the same if ammonium chloride
(NH
Cl) is added to the ammonia solution? Explain.
4
(c) In which direction does the equilibrium shift when HCl
is added to aqueous solution of ammonia?
(aq)
+
Does the equilibrium concentration of NH
increase, decrease, or stay the same if some HCl
is
4
(aq)
added to the solution? Explain.
1
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5