Vfc Program Vaccine Inventory Form
ADVERTISEMENT
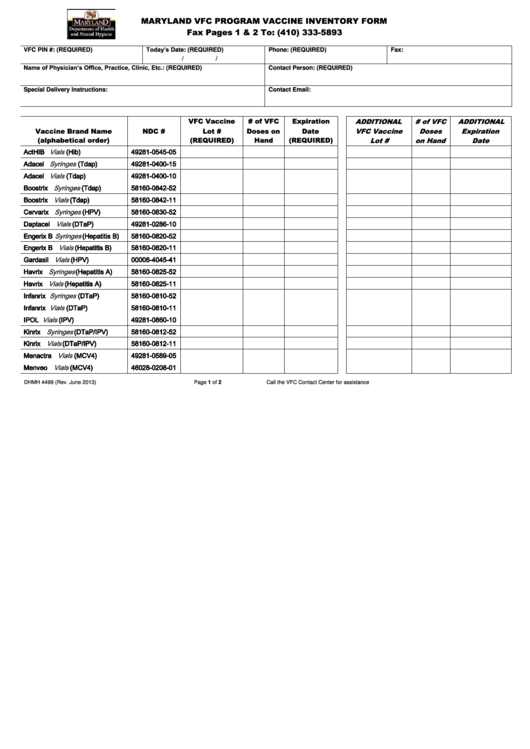
MARYLAND VFC PROGRAM VACCINE INVENTORY FORM
Fax Pages 1 & 2 To: (410) 333-5893
Today’s Date: (REQUIRED)
VFC PIN #: (REQUIRED)
Phone: (REQUIRED)
Fax:
/
/
Name of Physician’s Office, Practice, Clinic, Etc.: (REQUIRED)
Contact Person: (REQUIRED)
Special Delivery Instructions:
Contact Email:
ADDITIONAL
# of VFC
ADDITIONAL
VFC Vaccine
# of VFC
Expiration
VFC Vaccine
Doses
Expiration
Vaccine Brand Name
NDC #
Lot #
Doses on
Date
Lot #
on Hand
Date
(alphabetical order)
(REQUIRED)
Hand
(REQUIRED)
ActHIB
Vials
(Hib)
49281-0545-05
Adacel
Syringes
(Tdap)
49281-0400-15
Vials
Adacel
(Tdap)
49281-0400-10
Boostrix
Syringes
(Tdap)
58160-0842-52
Vials
Boostrix
(Tdap)
58160-0842-11
Syringes
Cervarix
(HPV)
58160-0830-52
Vials
Daptacel
(DTaP)
49281-0286-10
Syringes
Engerix B
(Hepatitis B)
58160-0820-52
Vials
Engerix B
(Hepatitis B)
58160-0820-11
Vials
Gardasil
(HPV)
00006-4045-41
Syringes
Havrix
(Hepatitis A)
58160-0825-52
Vials
Havrix
(Hepatitis A)
58160-0825-11
Syringes
Infanrix
(DTaP)
58160-0810-52
Vials
Infanrix
(DTaP)
58160-0810-11
IPOL
Vials
(IPV)
49281-0860-10
Kinrix
Syringes
(DTaP/IPV)
58160-0812-52
Kinrix
Vials
(DTaP/IPV)
58160-0812-11
Menactra
Vials
(MCV4)
49281-0589-05
Menveo
Vials
(MCV4)
46028-0208-01
DHMH 4499 (Rev. June 2013)
Page 1 of 2
Call the VFC Contact Center for assistance
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Legal
 1
1 2
2








