Elimination Reactions And Alkene Synthesis - Organic Chemistry Worksheet With Answers
ADVERTISEMENT
ORGANIC CHEMISTRY I – PRACTICE EXERCISE
Elimination Reactions and Alkene Synthesis
1) One of the products that results when 1-bromo-2,2-dimethylcyclopentane is heated in ethanol
is shown below. Give a mechanism by which it is formed and give the name of this mechanism.
CH
3
CH
3
2) Provide the structure of the major organic product in the following reaction.
CH
3
NaOCH
3
CH
OH
Br
3
H
D
3) Provide the structure of the major organic product from following reaction.
H
NaOCH
3
CH
OH
3
CH
3
H
Br
4) Which diastereomer of 1-bromo-4-t-butylcyclohexane, the cis or the trans, undergoes
elimination more rapidly when treated with sodium ethoxide? Explain your answer.
5) Provide the structure of the major organic product from the following reaction.
Br
KI
H
C
Br
3
6) When 1-iodo-1-methylcyclohexane is treated with NaOCH 2 CH 3 as the base, the more highly
substituted alkene product predominates. When KOC(CH 3 ) 3 is used as the base, the less highly
substituted alkene predominates. Give the structures of the two products and offer an
explanation.
7) Which of the following statements apply to E1 reactions of alkyl halides? Choose as many as
necessary.
I.
Rate = k[base]
II.
Rate = k[base][RX]
III.
Rate = k[RX]
IV.
The reactions occur in two or more distinct steps.
V.
Rearrangements are sometimes seen.
8) What is Saytzeff's rule?
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2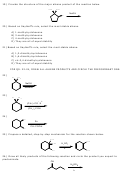 3
3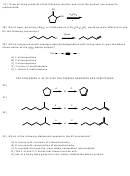 4
4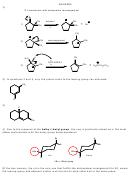 5
5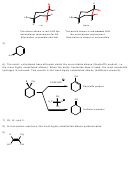 6
6 7
7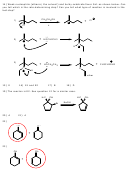 8
8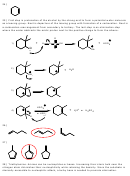 9
9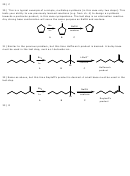 10
10








