Chemistry Chem 125, 126, 130 Homework 4 With Answers - University Of Michigan, Fall 2006
ADVERTISEMENT
Chem 125, 126, 130
Fall 2006
HW 4
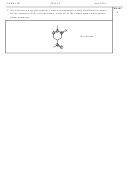
1) For each of the following: a) find the number of valence electrons; b) draw the
Lewis Structure (include all resonance structures); c) calculate the formal charge
for each atom in each molecule and add it to the Lewis Structure; d) fill in the
remainder of the information requested in the table.
a) SO
b) SO
2
3
-
-
18 e
26 e
-1
-1
O
O
-1
+1
-1
+1
O
S
O
O
S
O
-1
-1
S
S
O
O
O
+2
O
+2
S
O
O
O
-1
-1
S
+2
O
O
Name:
sulfur dioxide
Name:
sulfur trioxide
Electron pair geometry:
triangular
Electron pair
geometry:triangular planar
planar
Molecular geometry:
bent
Molecular geometry:
triangular planar
-2
-2
c) SO
d) CO
3
3
-
-
26 e
24 e
-2
-2
-1
-1
-2
O
O
-1
O
S
-1
-1
C
C
-1
-1
O
O
O
O
S
O
O
O
O
O
+1
O
-2
-1
-1
C
O
O
Name:
sulfite
Name:
carbonate
Electron pair geometry:
tetrahedral
Electron pair geometry:
triangular
planar
Molecular
geometry:triangular pyramidal
Molecular geometry:
triangular planar
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3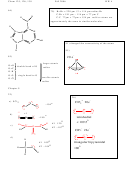 4
4