Bonding Concepts Worksheet - Unit 8/9, Michalek Page 11
ADVERTISEMENT
AP Chemistry - Michalek
11
Unit 08 Bonding Concepts
9.1 Hybridization and the Localized Electron Model
Read and outline 9.1
Define
Hybridization
3
sp
hybridization
hybrid orbitals
2
sp
hybridization
sigma bonds
pi bonds
sp hybridization
3
dsp
hybridization
Methane
2
3
d
sp
hybridization
9.1 Notes
Hybridization
The mixing of the native atomic orbitals to form
special orbitals for bonding.
3
With sp
orbitals, the atomic orbitals of s and p
reshape to form the tetrahedral shape.
Whenever a set of equivalent tetrahedral atomic orbitals is
required by an atom, this model assumes that the atom adopts a
3
3
set of sp
orbitals; the atom becomes sp
hybridized.
Example
Describe the bonding in the ammonia using the localized electron
model.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9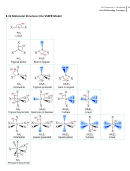 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30








