Bonding Concepts Worksheet - Unit 8/9, Michalek Page 23
ADVERTISEMENT
AP Chemistry - Michalek
23
Unit 08 Bonding Concepts
8.7 The Covalent Chemical Bond: A Model—Read this section
Chemical bonds can be viewed as a force that causes a group of atoms to behave as a unit. Bonds result from the
tendency of a system to seek its lowest possible energy.
8. 8 Covalent Bond Energies and Chemical Reactions
Read and outline 8.8
Define
Single Bond
Double Bond
Triple Bond
What is the equation for ΔH
from unit 6?
f
What process is the formation of a bond?
When a bind breaks is energy absorbed or releases
from the molecule? What process is this?
Bond Type
Bonds
Shared electron pairs
Single
1
1
Double
2
2
Triple
3
3
ΔH
= nBE reactant – nBE products
Bond energies
Example 8.8a
Using the bond energies Calculate ΔH for the reaction
of methane with chlorine and Fluorine for give CF
Cl
2
2
with hydrofluoric and hydrochloric acid.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9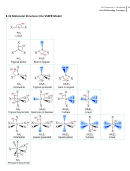 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30








