Bonding Concepts Worksheet - Unit 8/9, Michalek Page 30
ADVERTISEMENT
AP Chemistry - Michalek
30
Unit 08 Bonding Concepts
Worksheet 9.01
Name ___________________________________
MOs and Bond Order
1. Use the molecular orbital model, write electron configurations for the following diatomic species and calculate
the bond orders. Are they paramagnetic or diamagnetic? Li
, O
, C
2
2
2
2. Use the molecular orbital model, write electron configurations for the following diatomic species and calculate
+
+2
the bond orders. Are they paramagnetic or diamagnetic? CO, CO
, CO
.
3. Place the species in number two in order of increasing bond length and increasing energy. Explain your answer.
-
4. Describe the bonding in O
molecule and the NO
ion using the localized electron model. How would the
3
2
molecular orbital model describe the pi bonding in these two species?
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9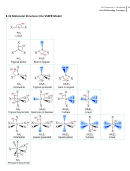 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30








