Bonding Concepts Worksheet - Unit 8/9, Michalek Page 4
ADVERTISEMENT
AP Chemistry - Michalek
4
Unit 08 Bonding Concepts
8.2 Electronegativity
Read and outline 8.2
Define
Electronegativity
8.2 Notes
Electronegativity is the ability of an atom to attract shared electrons to itself.
Expected H-X bond energy = H-H bond energy + X-X bond energy
2
∆ = (H-X) act – (H-X)exp
When the bond strength is greater the actual bond energy will be larger than the expected.
Example
Order the following bonds according to polarity: H-H, O-H, Cl- H, S-H, and F-H.
8.5 Formation of Binary Ionic Compounds
Read and outline 8.5
Define
Lattice Energy
Describe the steps to form a generic ionic bond. (This means don’t use any symbols of chemicals)
1.
2.
3.
4.
5.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9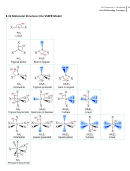 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26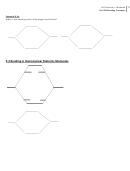 27
27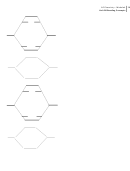 28
28 29
29 30
30








