Chapter 12 - Structure Determination: Mass Spectrometry And Infrared Spectroscopy Page 2
ADVERTISEMENT
Principles of Spectroscopy
The relationship between energy of light E, and its
frequency, is given by the equation:
E = h
The equation says that there is a direct relationship
between the frequency of light and its energy: the
higher the frequency, the higher the energy.
The proportionality constant between energy and
frequency is known as Plank’s constant, h.
2
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21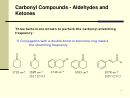 22
22 23
23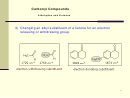 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34 35
35 36
36 37
37 38
38 39
39 40
40 41
41 42
42 43
43 44
44








