Stoichiometry Worksheet
ADVERTISEMENT
Name: _______________________________________________________
Video 8.1 – Intro to Stoichiometry
What is stoichiometry? - Predicting quantities in a chemical reaction.
You can predict the number of moles, grams, Liters of something in a chemical reaction.
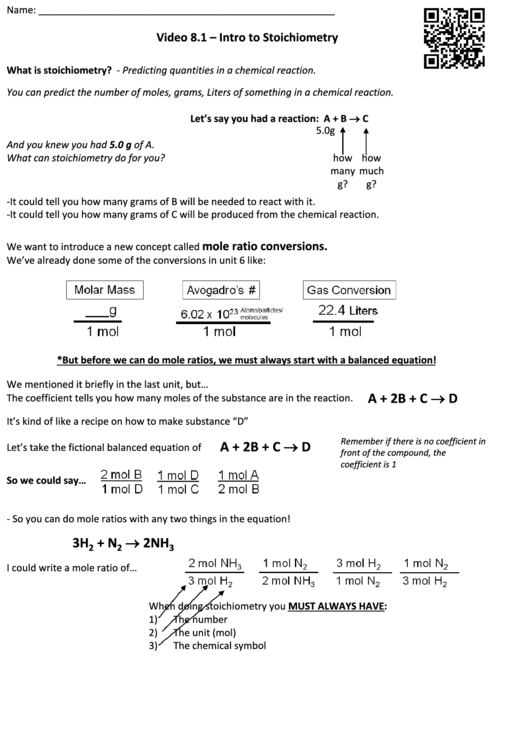
Let’s say you had a reaction: A + B C
5.0g
And you knew you had 5.0 g of A.
What can stoichiometry do for you?
how
how
many
much
g?
g?
-It could tell you how many grams of B will be needed to react with it.
-It could tell you how many grams of C will be produced from the chemical reaction.
mole ratio conversions.
We want to introduce a new concept called
We’ve already done some of the conversions in unit 6 like:
*But before we can do mole ratios, we must always start with a balanced equation!
We mentioned it briefly in the last unit, but…
A + 2B + C D
The coefficient tells you how many moles of the substance are in the reaction.
It’s kind of like a recipe on how to make substance “D”
Remember if there is no coefficient in
A + 2B + C D
Let’s take the fictional balanced equation of
front of the compound, the
coefficient is 1
So we could say…
- So you can do mole ratios with any two things in the equation!
2NH
3H
+ N
2
2
3
I could write a mole ratio of…
When doing stoichiometry you MUST ALWAYS HAVE:
1)
The number
2)
The unit (mol)
3)
The chemical symbol
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4








