Recrystallization
ADVERTISEMENT
1
Experiment 1: Recrystallization
Read pp 221-231 and 234-235, Chapter 15, in LTOC. The theory and technique
of the purification of solid compounds by recrystallization is fully explained in these
pages. Read also pp 73-74 (6.1) to learn about boiling stones and sticks, and page 75 to
review the use of hot plates for heating flat-bottomed containers.
Part 1. Recrystallization of Pure Phthalic Acid
In the first of today's procedures, you will
O
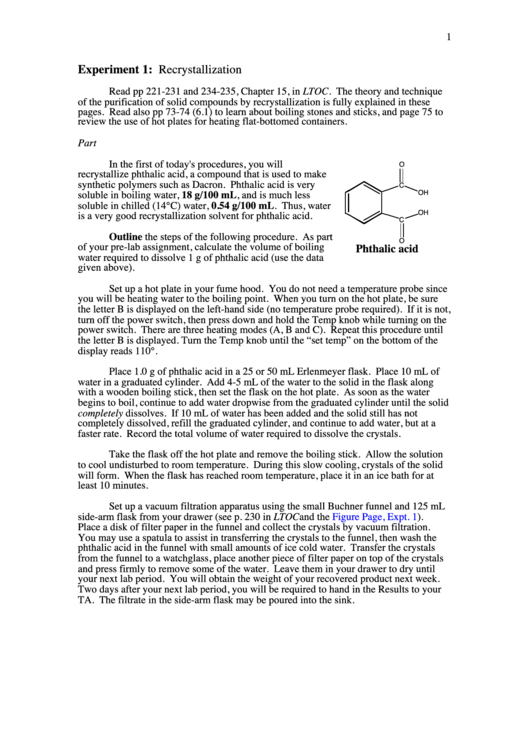
recrystallize phthalic acid, a compound that is used to make
synthetic polymers such as Dacron. Phthalic acid is very
C
OH
soluble in boiling water, 18 g/100 mL, and is much less
soluble in chilled (14°C) water, 0.54 g/100 mL. Thus, water
OH
is a very good recrystallization solvent for phthalic acid.
C
Outline the steps of the following procedure. As part
O
of your pre-lab assignment, calculate the volume of boiling
Phthalic acid
water required to dissolve 1 g of phthalic acid (use the data
given above).
Set up a hot plate in your fume hood. You do not need a temperature probe since
you will be heating water to the boiling point. When you turn on the hot plate, be sure
the letter B is displayed on the left-hand side (no temperature probe required). If it is not,
turn off the power switch, then press down and hold the Temp knob while turning on the
power switch. There are three heating modes (A, B and C). Repeat this procedure until
the letter B is displayed. Turn the Temp knob until the “set temp” on the bottom of the
display reads 110°.
Place 1.0 g of phthalic acid in a 25 or 50 mL Erlenmeyer flask. Place 10 mL of
water in a graduated cylinder. Add 4-5 mL of the water to the solid in the flask along
with a wooden boiling stick, then set the flask on the hot plate. As soon as the water
begins to boil, continue to add water dropwise from the graduated cylinder until the solid
completely dissolves. If 10 mL of water has been added and the solid still has not
completely dissolved, refill the graduated cylinder, and continue to add water, but at a
faster rate. Record the total volume of water required to dissolve the crystals.
Take the flask off the hot plate and remove the boiling stick. Allow the solution
to cool undisturbed to room temperature. During this slow cooling, crystals of the solid
will form. When the flask has reached room temperature, place it in an ice bath for at
least 10 minutes.
Set up a vacuum filtration apparatus using the small Buchner funnel and 125 mL
side-arm flask from your drawer (see p. 230 in LTOC and the
Figure Page, Expt.
1).
Place a disk of filter paper in the funnel and collect the crystals by vacuum filtration.
You may use a spatula to assist in transferring the crystals to the funnel, then wash the
phthalic acid in the funnel with small amounts of ice cold water. Transfer the crystals
from the funnel to a watchglass, place another piece of filter paper on top of the crystals
and press firmly to remove some of the water. Leave them in your drawer to dry until
your next lab period. You will obtain the weight of your recovered product next week.
Two days after your next lab period, you will be required to hand in the Results to your
TA. The filtrate in the side-arm flask may be poured into the sink.
ADVERTISEMENT
0 votes
Related Articles
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4