Bonding Types Worksheet
ADVERTISEMENT
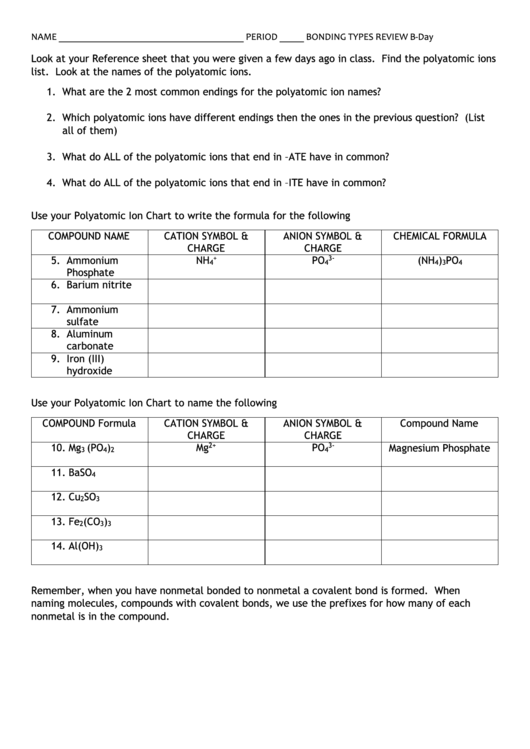
NAME ______________________________________ PERIOD _____ BONDING TYPES REVIEW B-Day
Look at your Reference sheet that you were given a few days ago in class. Find the polyatomic ions
list. Look at the names of the polyatomic ions.
1. What are the 2 most common endings for the polyatomic ion names?
2. Which polyatomic ions have different endings then the ones in the previous question? (List
all of them)
3. What do ALL of the polyatomic ions that end in –ATE have in common?
4. What do ALL of the polyatomic ions that end in –ITE have in common?
Use your Polyatomic Ion Chart to write the formula for the following
COMPOUND NAME
CATION SYMBOL &
ANION SYMBOL &
CHEMICAL FORMULA
CHARGE
CHARGE
+
3-
5. Ammonium
NH
PO
(NH
)
PO
4
4
4
3
4
Phosphate
6. Barium nitrite
7. Ammonium
sulfate
8. Aluminum
carbonate
9. Iron (III)
hydroxide
Use your Polyatomic Ion Chart to name the following
COMPOUND Formula
CATION SYMBOL &
ANION SYMBOL &
Compound Name
CHARGE
CHARGE
10. Mg
(PO
)
Mg
2+
PO
3-
Magnesium Phosphate
3
4
2
4
11. BaSO
4
12. Cu
SO
2
3
13. Fe
(CO
)
2
3
3
14. Al(OH)
3
Remember, when you have nonmetal bonded to nonmetal a covalent bond is formed. When
naming molecules, compounds with covalent bonds, we use the prefixes for how many of each
nonmetal is in the compound.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2








