Lewis Dot Structure Practice
ADVERTISEMENT
Student #: ________
Lewis Dot Structure Practice
Block:
________
Fall 2012 Chemistry I – Mr. Patel
Name ___________________________________________________
Date ______________________
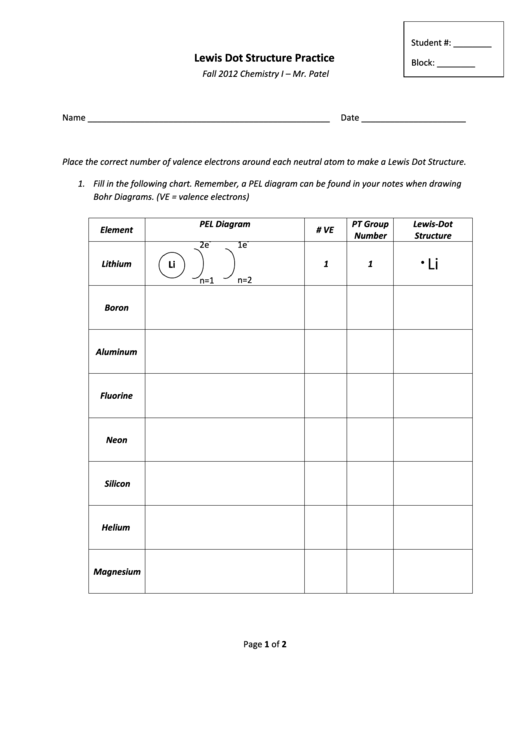
Place the correct number of valence electrons around each neutral atom to make a Lewis Dot Structure.
1. Fill in the following chart. Remember, a PEL diagram can be found in your notes when drawing
Bohr Diagrams. (VE = valence electrons)
PEL Diagram
PT Group
Lewis-Dot
Element
# VE
Number
Structure
-
-
2e
1e
Li
Lithium
1
1
Li
n=1
n=2
Boron
Aluminum
Fluorine
Neon
Silicon
Helium
Magnesium
Page 1 of 2
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2








