Advanced Chemistry Formulas Handout
ADVERTISEMENT
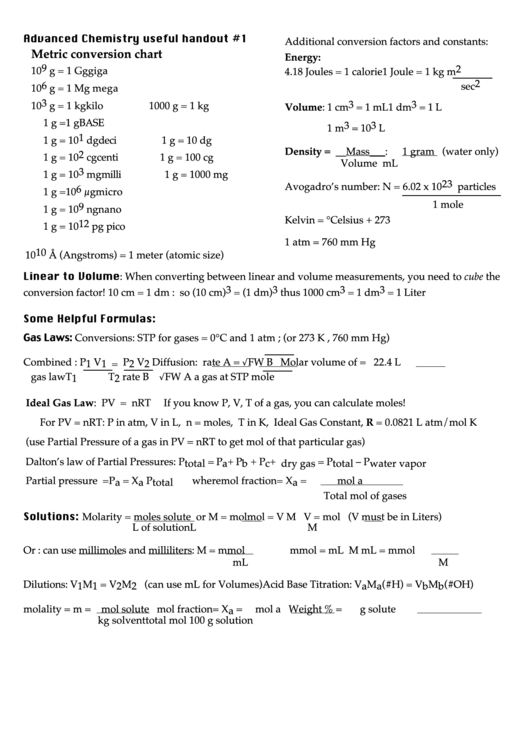
Advanced Chemistry useful handout #1
Additional conversion factors and constants:
Metric conversion chart
Energy:
10 9 g =
1 Joule = 1 kg m 2
1 Gg
giga
4.18 Joules = 1 calorie
sec 2
10 6 g =
1 Mg
mega
10 3 g =
Volume: 1 cm 3 = 1 mL
1 dm 3 = 1 L
1 kg
kilo
1000 g = 1 kg
1 g =
1 g
BASE
1 m 3 = 10 3 L
10 1 dg
1 g =
deci
1 g = 10 dg
Density = __Mass___:
1 gram (water only)
10 2 cg
1 g =
centi
1 g = 100 cg
Volume
mL
10 3 mg
1 g =
milli
1 g = 1000 mg
Avogadro’s number: N = 6.02 x 10 23 particles
10 6 µg
1 g =
micro
1 mole
10 9 ng
1 g =
nano
Kelvin = °Celsius + 273
10 12 pg
1 g =
pico
1 atm = 760 mm Hg
10 10 Å (Angstroms) = 1 meter (atomic size)
Linear to Volume: When converting between linear and volume measurements, you need to cube the
conversion factor! 10 cm = 1 dm : so (10 cm) 3 = (1 dm) 3 thus 1000 cm 3 = 1 dm 3 = 1 Liter
Some Helpful Formulas:
Gas Laws: Conversions: STP for gases = 0°C and 1 atm ; (or 273 K , 760 mm Hg)
Combined : P 1 V 1 = P 2 V 2
Diffusion: rate A = √FW B
Molar volume of = 22.4 L
gas law
T 1
T 2
rate B √FW A
a gas at STP
mole
Ideal Gas Law: PV = nRT
If you know P, V, T of a gas, you can calculate moles!
For PV = nRT: P in atm, V in L, n = moles, T in K, Ideal Gas Constant, R = 0.0821 L atm/mol K
(use Partial Pressure of a gas in PV = nRT to get mol of that particular gas)
Dalton’s law of Partial Pressures: P total = P a + P b + P c + ....
P dry gas = P total – P water vapor
Partial pressure = P a = X a P total
where mol fraction= X a =
mol a
Total mol of gases
Solutions: Molarity = moles solute or
M = mol
mol = V M
V = mol (V must be in Liters)
L of solution
L
M
Or : can use millimoles and milliliters: M = mmol
mmol = mL M
mL = mmol
mL
M
Dilutions: V 1 M 1 = V 2 M 2 (can use mL for Volumes)
Acid Base Titration: V a M a (#H) = V b M b (#OH)
molality = m = mol solute
mol fraction= X a =
mol a
Weight % =
g solute
kg solvent
total mol
100 g solution
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2








