Periodic Table Groups Page 2
ADVERTISEMENT
Investigative Science –P
T
Friday September 4th, 2015
ERIODIC
ABLE GROUPS
Perry High School
Notebook pages: 72-73
Mr. Pomerantz_________________________________________________________________________Page 2 of 4
Sub-segments of the groups:
Post-transition metals – this segment includes lead and aluminum. These metals usually are soft, have low boiling
points and poor electrical conductivity. Lead happens to be the heaviest stable element.
Metalloids – These elements are the boarder between metals and nonmetals. Some metalloids combine the properties of
metals and non-metals. Two examples are silicon and germanium: they are generally poor conductors of electricity
(like nonmetals) however under special condition these two elements become excellent conductors of electricity (like
metals). Having multiple properties makes metalloids (like silicon) very useful in computer and microprocessors.
Non-metals: These elements do not conduct electricity or heat well (if at all). These elements are brittle when solid,
they cannot be rolled into wire or pounded into sheets (like copper wire or aluminum foil), and they have no luster (or
shine). Non-metals will be gases (like oxygen) or solids (like carbon) at room temperature.
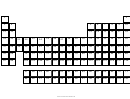
This worksheet will help you understand how the periodic table is arranged. Follow the directions below.
1.
Color the square for Hydrogen light green.
2.
Color the Alkali metals orange.
3.
Color the Alkaline-Earth metals gray.
4.
Color the Transition metals brown.
Color Al, Ga, In, Tl, Sn, Pb, and Bi yellow. These are “post-transition” metals.
5.
6.
Color B, Si, Ge, As, Sb, Te, and Po red. These are the Metalloids.
7.
Color C, N, O, P, S, and Se dark blue. These are Nonmetals.
8.
Color the Halogens light pink.
9.
Color the Nobel gasses light blue.
10. Color all the Lanthanides dark green.
11. Color all the Actinides dark green.
12. Outline groups (vertical columns) 1, 2, 13, 14, 15, 16, 17, and 18 with a dark black line. Above the first
element in each group, write the number of valence electrons elements in each group has.
13. When you are finished, make a key that indicate what each color identifies.
14. Repeat the direction above with the blank periodic table making one modification: outline, do not color-in each
segment of the periodic table. Do not write the elements in the boxes.
15. Find two words from the reading (above and front of the sheet) that are specific descriptors of the elements in
each outlined segment. Write these words in the open space.
16. Think of two descriptive and identifying pictures for each outlined segment. Draw these pictures in the
appropriate segment.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Miscellaneous
 1
1 2
2 3
3 4
4