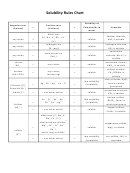
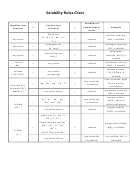
Solubility Rules Chart
ADVERTISEMENT
CHEM
111,
Gallagher
Fall
2009
Solubility Rules
How to determine if an ionic compound is soluble or insoluble:
• Identify the two ions
• Check the “solubility rules”
o Soluble ions with no “exceptions” never form precipitates
o If one of the ions is insoluble, the compound is insoluble
o Make sure to check for “exceptions”
Example: K
CO
2
3
o If a reaction product is insoluble, it will form a precipitate
Are these compounds soluble or insoluble?
1. NaNO
7. K
PO
3
3
4
2. FeCl
8. Fe
(PO
)
3
3
4
2
3. Fe(OH)
9. PbCl
3
2
4. BaSO
10. FeSO
4
4
5. AgNO
11. (NH
)
S
3
4
2
6. AgCl
12. PbS
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1