Some Solubility Rules For Common Salts And Bases
ADVERTISEMENT
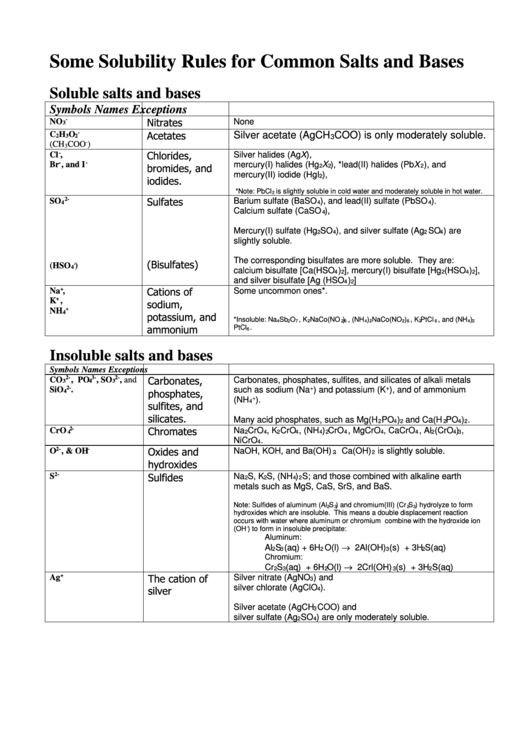
Some Solubility Rules for Common Salts and Bases
Soluble salts and bases
Symbols
Names
Exceptions
-
None
NO
Nitrates
3
-
C
H
O
Silver acetate (AgCH
COO) is only moderately soluble.
Acetates
2
3
2
3
-
(CH
COO
)
3
-
Silver halides (AgX),
Cl
,
Chlorides,
-
-
mercury(I) halides (Hg
X
), *lead(II) halides (PbX
), and
Br
, and I
2
2
2
bromides, and
mercury(II) iodide (HgI
),
2
iodides.
*Note: PbCl
is slightly soluble in cold water and moderately soluble in hot water.
2
2-
Barium sulfate (BaSO
), and lead(II) sulfate (PbSO
).
SO
Sulfates
4
4
4
Calcium sulfate (CaSO
),
4
Mercury(I) sulfate (Hg
SO
), and silver sulfate (Ag
SO
) are
2
4
2
4
slightly soluble.
The corresponding bisulfates are more soluble. They are:
(Bisulfates)
-
(HSO
)
4
calcium bisulfate [Ca(HSO
)
], mercury(I) bisulfate [Hg
(HSO
)
],
4
2
2
4
2
and silver bisulfate [Ag (HSO
)
]
4
2
+
Na
,
Some uncommon ones*.
Cations of
+
K
,
sodium,
+
NH
4
potassium, and
*Insoluble: Na
Sb
O
, K
NaCo(NO
)
, (NH
)
NaCo(NO
)
, K
PtCl
, and (NH
)
4
2
7
2
2
6
4
2
2
6
2
6
4
2
PtCl
.
ammonium
6
Insoluble salts and bases
Symbols
Names
Exceptions
2-
3-
2-
Carbonates, phosphates, sulfites, and silicates of alkali metals
CO
, PO
, SO
, and
Carbonates,
3
4
3
2-
+
+
SiO
.
such as sodium (Na
) and potassium (K
), and of ammonium
4
phosphates,
+
(NH
).
4
sulfites, and
silicates.
Many acid phosphates, such as Mg(H
PO
)
and Ca(H
PO
)
.
2
4
2
2
4
2
2-
Na
CrO
, K
CrO
, (NH
)
CrO
, MgCrO
, CaCrO
, Al
(CrO
)
,
CrO
Chromates
4
2
4
2
4
4
2
4
4
4
2
4
3
NiCrO
.
4
2-
-
NaOH, KOH, and Ba(OH)
. Ca(OH)
is slightly soluble.
O
, & OH
Oxides and
2
2
hydroxides
2-
Na
S, K
S, (NH
)
S; and those combined with alkaline earth
S
Sulfides
2
2
4
2
metals such as MgS, CaS, SrS, and BaS.
Note: Sulfides of aluminum (Al
S
) and chromium(III) (Cr
S
) hydrolyze to form
2
3
2
3
hydroxides which are insoluble. This means a double displacement reaction
occurs with water where aluminum or chromium combine with the hydroxide ion
-
(OH
) to form in insoluble precipitate:
Aluminum:
Al
S
(aq) + 6H
O(l) → 2Al(OH)
(s) + 3H
S(aq)
2
3
2
3
2
Chromium:
Cr
S
(aq) + 6H
O(l) → 2Crl(OH)
(s) + 3H
S(aq)
2
3
2
3
2
+
Silver nitrate (AgNO
) and
Ag
The cation of
3
silver chlorate (AgClO
).
4
silver
Silver acetate (AgCH
COO) and
3
silver sulfate (Ag
SO
) are only moderately soluble.
2
4
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1








