Solubility Of Ammonia In Aqueous Salt Solution At 25c
ADVERTISEMENT
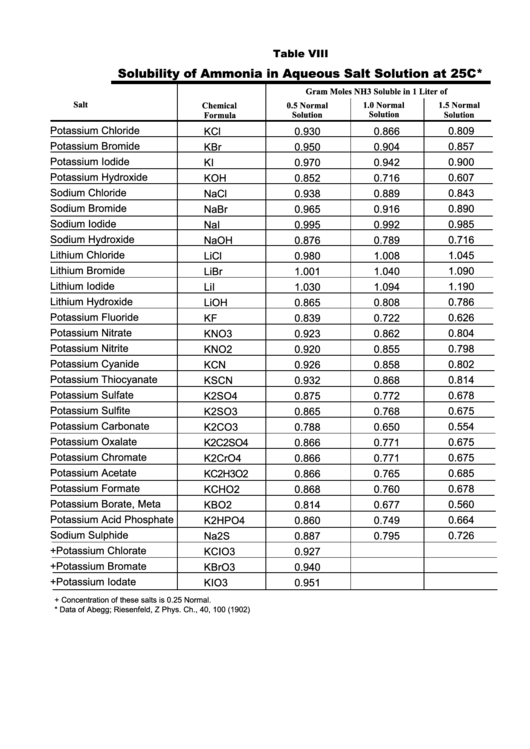
Table VIII
Solubility of Ammonia in Aqueous Salt Solution at 25C*
Gram Moles NH3 Soluble in 1 Liter of
Salt
1.0 Normal
1.5 Normal
Chemical
0.5 Normal
Solution
Solution
Solution
Formula
Potassium Chloride
0.809
KCl
0.930
0.866
Potassium Bromide
0.857
KBr
0.950
0.904
Potassium Iodide
0.942
0.900
KI
0.970
Potassium Hydroxide
KOH
0.852
0.716
0.607
Sodium Chloride
0.843
NaCl
0.938
0.889
Sodium Bromide
0.890
NaBr
0.965
0.916
Sodium Iodide
0.992
0.985
NaI
0.995
Sodium Hydroxide
NaOH
0.876
0.789
0.716
Lithium Chloride
1.045
LiCl
0.980
1.008
Lithium Bromide
1.090
LiBr
1.001
1.040
Lithium Iodide
1.094
1.190
LiI
1.030
Lithium Hydroxide
LiOH
0.865
0.808
0.786
Potassium Fluoride
0.626
KF
0.839
0.722
Potassium Nitrate
0.804
KNO3
0.923
0.862
Potassium Nitrite
0.855
0.798
KNO2
0.920
Potassium Cyanide
KCN
0.926
0.858
0.802
Potassium Thiocyanate
0.814
KSCN
0.932
0.868
Potassium Sulfate
0.678
K2SO4
0.875
0.772
Potassium Sulfite
0.768
0.675
K2SO3
0.865
Potassium Carbonate
K2CO3
0.788
0.650
0.554
Potassium Oxalate
0.675
K2C2SO4
0.866
0.771
Potassium Chromate
0.675
K2CrO4
0.866
0.771
Potassium Acetate
0.765
0.685
KC2H3O2
0.866
Potassium Formate
KCHO2
0.868
0.760
0.678
Potassium Borate, Meta
0.560
KBO2
0.814
0.677
Potassium Acid Phosphate
0.664
K2HPO4
0.860
0.749
Sodium Sulphide
0.795
0.726
Na2S
0.887
+Potassium Chlorate
KCIO3
0.927
+Potassium Bromate
KBrO3
0.940
+Potassium Iodate
KIO3
0.951
+ Concentration of these salts is 0.25 Normal.
* Data of Abegg; Riesenfeld, Z Phys. Ch., 40, 100 (1902)
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1








