Practice Problems Lewis Structures
ADVERTISEMENT
Practice Problems
2. Draw the Lewis dot structures for each of the following molecules:
a. H
S
c.
SO
2
3
b. CH
Br
d. HCN
2
2
3. Draw the Lewis dot structure for each of the following polyatomic ions:
–3
+
a. NH
c. PO
4
4
–
2–
b. NO
d. CO
3
3
4. For the following molecules or ions (where the central atom is underlined):
i. Draw the Electron dot structure.
ii. Determine the shape of the molecule.
iii. Determine the approximate bond angles.
a. CH
F
b. OF
2
2
2
CHM 130 Chapter 12
page 1 of 4
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1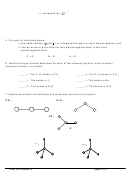 2
2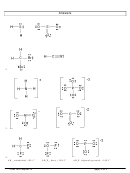 3
3 4
4








