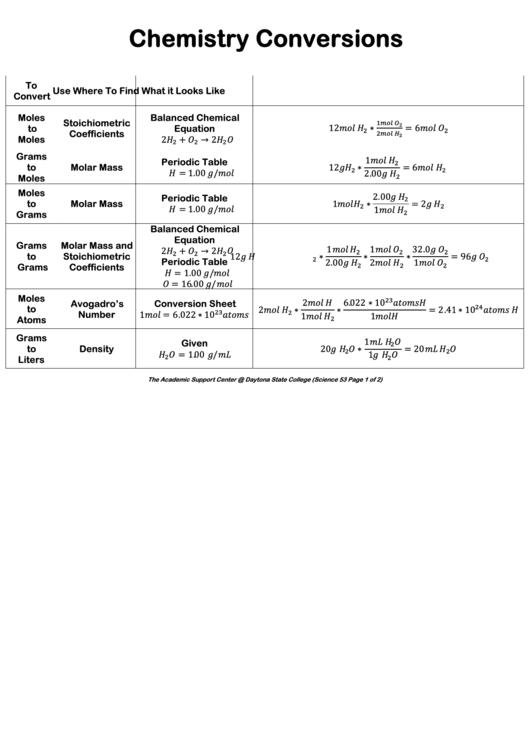
Chemistry Conversions
ADVERTISEMENT
Chemistry Conversions
To
Use
Where To Find
What it Looks Like
Convert
Moles
Balanced Chemical
Stoichiometric
12
∗
= 6
to
Equation
Coefficients
Moles
2
+
→ 2
Grams
1
Periodic Table
to
Molar Mass
12
∗
= 6
= 1.00 /
2.00
Moles
Moles
2.00
Periodic Table
to
Molar Mass
1
∗
= 2
= 1.00 /
1
Grams
Balanced Chemical
Equation
Grams
Molar Mass and
1
1
32.0
2
+
→ 2
to
Stoichiometric
12
∗
∗
∗
= 96
Periodic Table
2.00
2
1
Grams
Coefficients
= 1.00 /
= 16.00 /
Moles
2
6.022 ∗ 10
Conversion Sheet
Avogadro’s
to
2
∗
∗
= 2.41 ∗ 10
Number
1
= 6.022 ∗ 10
1
1
Atoms
Grams
1
Given
to
Density
20
∗
= 20
= 1.00 /
1
Liters
The Academic Support Center @ Daytona State College (Science 53 Page 1 of 2)
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2








