Percent Ionic Character Worksheet
ADVERTISEMENT
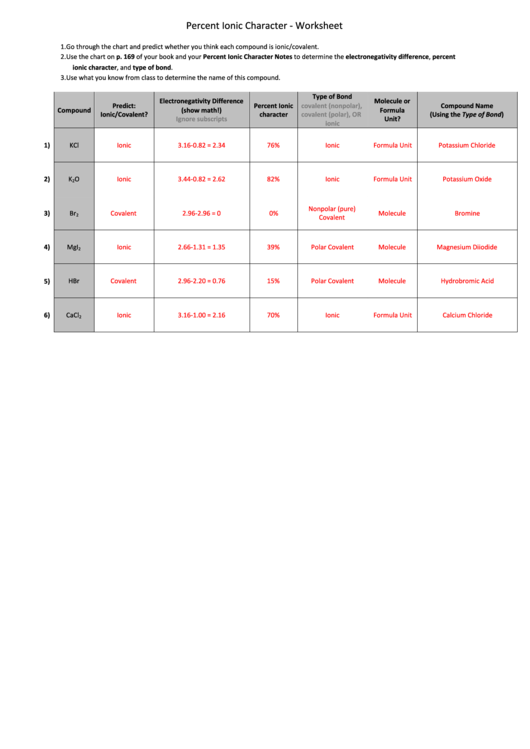
Percent Ionic Character ‐ Worksheet
1. Go through the chart and predict whether you think each compound is ionic/covalent.
2. Use the chart on p. 169 of your book and your Percent Ionic Character Notes to determine the electronegativity difference, percent
ionic character, and type of bond.
3. Use what you know from class to determine the name of this compound.
Type of Bond
Electronegativity Difference
Molecule or
Predict:
Percent Ionic
covalent (nonpolar),
Compound Name
Compound
(show math!)
Formula
Ionic/Covalent?
character
covalent (polar), OR
(Using the Type of Bond)
Ignore subscripts
Unit?
ionic
1)
KCl
Ionic
3.16‐0.82 = 2.34
76%
Ionic
Formula Unit
Potassium Chloride
2)
K
O
Ionic
3.44‐0.82 = 2.62
82%
Ionic
Formula Unit
Potassium Oxide
2
Nonpolar (pure)
3)
Br
Covalent
2.96‐2.96 = 0
0%
Molecule
Bromine
2
Covalent
4)
MgI
Ionic
2.66‐1.31 = 1.35
39%
Polar Covalent
Molecule
Magnesium Diiodide
2
5)
HBr
Covalent
2.96‐2.20 = 0.76
15%
Polar Covalent
Molecule
Hydrobromic Acid
6)
CaCl
Ionic
3.16‐1.00 = 2.16
70%
Ionic
Formula Unit
Calcium Chloride
2
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Miscellaneous
 1
1 2
2








