Chemical Elements
ADVERTISEMENT
4
The Essence of Mineralogy
Chemical Elements
arrangement is only systematic on the scale of
a few tetrahedra. So a glass is ordered only on
a local scale. In order to be a mineral, a materi-
As stated above, the elements are the basic
building blocks for minerals, and for that matter,
al must be made up of an orderly arrangement
of atoms on scales of tens of units put together.
all materials. There are about 90 known natural-
ly occurring elements. The good news is that
Before we continue, there are two important
only eight of these—oxygen, silicon, aluminum,
points to be made about number 4 and 5 above.
iron, calcium, sodium, potassium, and magne-
In many ways these two descriptions of a miner-
sium—compose over 98% of the Earth’s crust by
al form the basis for understanding minerals.
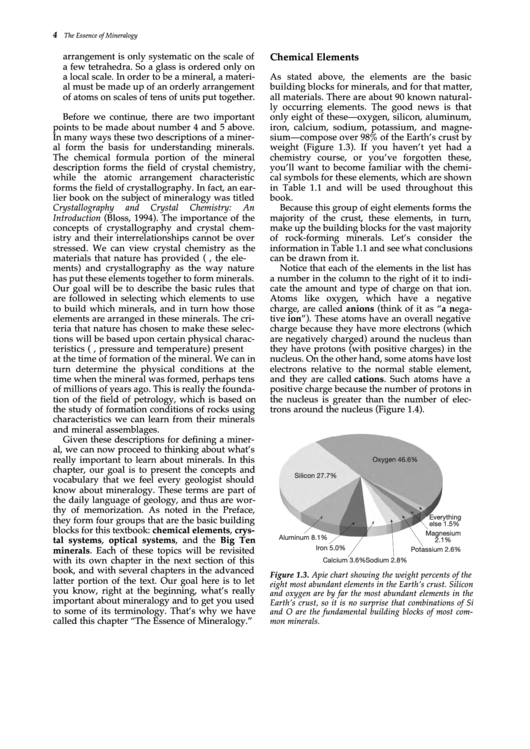
weight (Figure 1.3). If you haven’t yet had a
The chemical formula portion of the mineral
chemistry course, or you’ve forgotten these,
description forms the field of crystal chemistry,
you’ll want to become familiar with the chemi-
while the atomic arrangement characteristic
cal symbols for these elements, which are shown
forms the field of crystallography. In fact, an ear-
in Table 1.1 and will be used throughout this
lier book on the subject of mineralogy was titled
book.
Because this group of eight elements forms the
Crystallography
and
Crystal
Chemistry:
An
Introduction (Bloss, 1994). The importance of the
majority of the crust, these elements, in turn,
concepts of crystallography and crystal chem-
make up the building blocks for the vast majority
istry and their interrelationships cannot be over
of rock-forming minerals. Let’s consider the
information in Table 1.1 and see what conclusions
stressed. We can view crystal chemistry as the
materials that nature has provided (i.e., the ele-
can be drawn from it.
ments) and crystallography as the way nature
Notice that each of the elements in the list has
has put these elements together to form minerals.
a number in the column to the right of it to indi-
Our goal will be to describe the basic rules that
cate the amount and type of charge on that ion.
are followed in selecting which elements to use
Atoms like oxygen, which have a negative
to build which minerals, and in turn how those
charge, are called anions (think of it as “a nega-
elements are arranged in these minerals. The cri-
tive ion”). These atoms have an overall negative
teria that nature has chosen to make these selec-
charge because they have more electrons (which
tions will be based upon certain physical charac-
are negatively charged) around the nucleus than
teristics (e.g., pressure and temperature) present
they have protons (with positive charges) in the
at the time of formation of the mineral. We can in
nucleus. On the other hand, some atoms have lost
turn determine the physical conditions at the
electrons relative to the normal stable element,
time when the mineral was formed, perhaps tens
and they are called cations. Such atoms have a
of millions of years ago. This is really the founda-
positive charge because the number of protons in
tion of the field of petrology, which is based on
the nucleus is greater than the number of elec-
the study of formation conditions of rocks using
trons around the nucleus (Figure 1.4).
characteristics we can learn from their minerals
and mineral assemblages.
Given these descriptions for defining a miner-
al, we can now proceed to thinking about what’s
really important to learn about minerals. In this
chapter, our goal is to present the concepts and
Oxygen 46.6%
vocabulary that we feel every geologist should
Silicon 27.7%
know about mineralogy. These terms are part of
the daily language of geology, and thus are wor-
thy of memorization. As noted in the Preface,
they form four groups that are the basic building
blocks for this textbook: chemical elements, crys-
Everything
else 1.5%
tal systems, optical systems, and the Big Ten
Magnesium
minerals. Each of these topics will be revisited
Aluminum 8.1%
2.1%
with its own chapter in the next section of this
Iron 5.0%
Potassium 2.6%
book, and with several chapters in the advanced
Calcium 3.6%
Sodium 2.8%
Figure 1.3. A pie chart showing the weight percents of the
latter portion of the text. Our goal here is to let
eight most abundant elements in the Earth’s crust. Silicon
you know, right at the beginning, what’s really
and oxygen are by far the most abundant elements in the
important about mineralogy and to get you used
Earth’s crust, so it is no surprise that combinations of Si
to some of its terminology. That’s why we have
and O are the fundamental building blocks of most com-
called this chapter “The Essence of Mineralogy.”
mon minerals.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4