Properties Of Water Lab - Student Report
ADVERTISEMENT
Name
Student Response sheet
Period
Properties of Water Lab
:
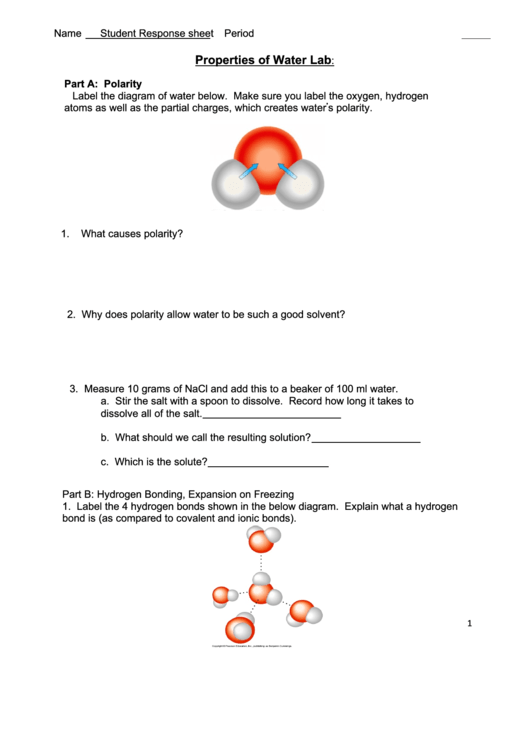
Part A: Polarity
Label the diagram of water below. Make sure you label the oxygen, hydrogen
atoms as well as the partial charges, which creates waterʼs polarity.
1.
What causes polarity?
2. Why does polarity allow water to be such a good solvent?
3. Measure 10 grams of NaCl and add this to a beaker of 100 ml water.
a. Stir the salt with a spoon to dissolve. Record how long it takes to
dissolve all of the salt.
b. What should we call the resulting solution?
c. Which is the solute?
Part B: Hydrogen Bonding, Expansion on Freezing
1. Label the 4 hydrogen bonds shown in the below diagram. Explain what a hydrogen
bond is (as compared to covalent and ionic bonds).
1
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4








